Research Articles
Ana M. López-Estévez, Y. Zhang, María Medel, Iker Arriaga, Lucía Sanjurjo, Cristian Huck-Iriart, Nicola G.A. Abrescia, María J. Vicent, Defang Ouyang, Dolores Torres, María José Alonso.”Engineering Hyaluronic Acid-based Nanoassemblies for Monoclonal Antibody Delivery – Design, Characterization, and Biological Insights”. Nano Research 2024. In Press [Nano Research]
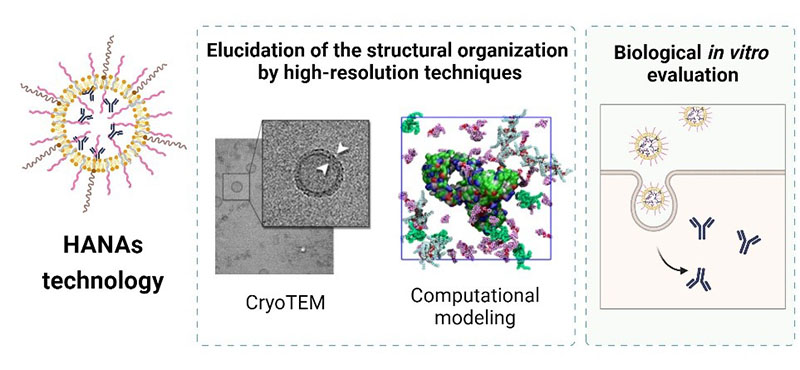 The current spotlight of cancer therapeutics is shifting towards personalized medicine with the widespread use of monoclonal antibodies (mAbs). Despite their increasing potential, mAbs have an intrinsic limitation related to their inability to cross cell membranes and reach intracellular targets. Nanotechnology offers promising solutions to overcome this limitation, however, formulation challenges remain. These challenges are the limited loading capacity (often insufficient to achieve clinical dosing), the complex formulation methods, and the insufficient characterization of mAb-loaded nanocarriers. Here, we present a new nanocarrier consisting of hyaluronic acid-based nanoassemblies (HANAs) specifically designed to entrap mAbs with a high efficiency and an outstanding loading capacity (50%, w/w). HANAs composed by an mAb, modified HA and phosphatidylcholine (PC) resulted in sizes of ~100 nm and neutral surface charge. Computational modeling identified the principal factors governing the high affinity of mAbs with the amphiphilic HA and PC. HANAs composition and structural configuration were analyzed using the orthogonal techniques cryogenic transmission electron microscopy (cryo-TEM), asymmetrical flow field-flow fractionation (AF4), and small-angle X-ray scattering (SAXS). These techniques provided evidence of the formation of core-shell nanostructures comprising an aqueous core surrounded by a bilayer consisting of phospholipids and amphiphilic HA. In vitro experiments in cancer cell lines and macrophages confirmed HANAs’ low toxicity and ability to transport mAbs to the intracellular space. The reproducibility of this assembling process at industrial-scale batch sizes and the long-term stability was assessed. In conclusion, these results underscore the suitability of HANAs technology to load and deliver biologicals, which holds promise for future clinical translation.
The current spotlight of cancer therapeutics is shifting towards personalized medicine with the widespread use of monoclonal antibodies (mAbs). Despite their increasing potential, mAbs have an intrinsic limitation related to their inability to cross cell membranes and reach intracellular targets. Nanotechnology offers promising solutions to overcome this limitation, however, formulation challenges remain. These challenges are the limited loading capacity (often insufficient to achieve clinical dosing), the complex formulation methods, and the insufficient characterization of mAb-loaded nanocarriers. Here, we present a new nanocarrier consisting of hyaluronic acid-based nanoassemblies (HANAs) specifically designed to entrap mAbs with a high efficiency and an outstanding loading capacity (50%, w/w). HANAs composed by an mAb, modified HA and phosphatidylcholine (PC) resulted in sizes of ~100 nm and neutral surface charge. Computational modeling identified the principal factors governing the high affinity of mAbs with the amphiphilic HA and PC. HANAs composition and structural configuration were analyzed using the orthogonal techniques cryogenic transmission electron microscopy (cryo-TEM), asymmetrical flow field-flow fractionation (AF4), and small-angle X-ray scattering (SAXS). These techniques provided evidence of the formation of core-shell nanostructures comprising an aqueous core surrounded by a bilayer consisting of phospholipids and amphiphilic HA. In vitro experiments in cancer cell lines and macrophages confirmed HANAs’ low toxicity and ability to transport mAbs to the intracellular space. The reproducibility of this assembling process at industrial-scale batch sizes and the long-term stability was assessed. In conclusion, these results underscore the suitability of HANAs technology to load and deliver biologicals, which holds promise for future clinical translation.
Inés Domingo-Ortí, Patricia Ferrer-Torres, Ana Armiñán, María J. Vicent*, Antonio Pineda-Lucena*, and Martina Palomino-Schätzlein.* “NMR-Based Mitochondria Metabolomic Profiling: A New Approach To Reveal Cancer-Associated Alterations”. Analytical Chemistry 2023;14;95(45):16539-16548. [PubMed][Anal. Chem.]
 Studying metabolism may assist in understanding the relationship between normal and dysfunctional mitochondrial activity and various diseases, such as neurodegenerative, cardiovascular, autoimmune, psychiatric, and cancer. Nuclear magnetic resonance-based metabolomics represents a powerful method to characterize the chemical content of complex samples and has been successfully applied to studying a range of conditions. However, an optimized methodology is lacking for analyzing isolated organelles, such as mitochondria. In this study, we report the development of a protocol to metabolically profile mitochondria from healthy, tumoral, and metastatic tissues. Encouragingly, this approach provided quantitative information about up to 45 metabolites in one comprehensive and robust analysis. Our results revealed significant differences between whole-cell and mitochondrial metabolites, which supports a more refined approach to metabolic analysis. We applied our optimized methodology to investigate aggressive and metastatic breast cancer in mouse tissues, discovering that lung mitochondria exhibit an altered metabolic fingerprint. Specific amino acids, organic acids, and lipids showed significant increases in levels when compared with mitochondria from healthy tissues. Our optimized methodology could promote a better understanding of the molecular mechanisms underlying breast cancer aggressiveness and mitochondrial-related diseases and support the optimization of new advanced therapies.
Studying metabolism may assist in understanding the relationship between normal and dysfunctional mitochondrial activity and various diseases, such as neurodegenerative, cardiovascular, autoimmune, psychiatric, and cancer. Nuclear magnetic resonance-based metabolomics represents a powerful method to characterize the chemical content of complex samples and has been successfully applied to studying a range of conditions. However, an optimized methodology is lacking for analyzing isolated organelles, such as mitochondria. In this study, we report the development of a protocol to metabolically profile mitochondria from healthy, tumoral, and metastatic tissues. Encouragingly, this approach provided quantitative information about up to 45 metabolites in one comprehensive and robust analysis. Our results revealed significant differences between whole-cell and mitochondrial metabolites, which supports a more refined approach to metabolic analysis. We applied our optimized methodology to investigate aggressive and metastatic breast cancer in mouse tissues, discovering that lung mitochondria exhibit an altered metabolic fingerprint. Specific amino acids, organic acids, and lipids showed significant increases in levels when compared with mitochondria from healthy tissues. Our optimized methodology could promote a better understanding of the molecular mechanisms underlying breast cancer aggressiveness and mitochondrial-related diseases and support the optimization of new advanced therapies.
O. Zagorodko*, T. Melnyk, V. J. Nebot, P. Y. W. Dankers, M. J. Vicent* “An Offset Patterned Cross-β Structure in Assemblies of C3-Symmetric Peptide Amphiphiles”. Chemistry. 2023;15:e202303194. [PubMed][Chemistry]
 Developing peptide-based materials with controlled morphology represents a critical theme of soft matter research. Herein, we report the formation of a novel, patterned cross-β structure formed by self-assembled C3-symmetric peptide amphiphiles based on diphenylalanine and benzene-1,3,5-tricarboxamide (BTA). The cross-β motif is an abundant structural element in amyloid fibrils and aggregates of fibril-forming peptides, including diphenylalanine. The incorporation of topological constraints on one edge of the diphenylalanine fragment limits the number of β-strands in β-sheets and leads to the creation of an unconventional offset patterned cross-β structure consisting of short 3×2 parallel β-sheets stabilized by phenylalanine zippers. In the reported assembly, two patterned cross-β structures bind parallel arrays of BTA stacks in a superstructure within a single-molecule thick nanoribbon. In addition to a threefold network of hydrogen bonds in the BTA stack, each molecule becomes simultaneously bound by hydrogen bonds of three β-sheets and four phenylalanine zippers. The diffuse layer of alkyl chains with terminal polar groups prevents merging of the nanoribbons and stabilizes cross-β-structure in water. Our results provide a simple approach to the incorporation of novel patterned cross-β motifs into supramolecular superstructures and shed light on the general mechanism of β-sheet formation in C3-symmetric peptide amphiphiles.
Developing peptide-based materials with controlled morphology represents a critical theme of soft matter research. Herein, we report the formation of a novel, patterned cross-β structure formed by self-assembled C3-symmetric peptide amphiphiles based on diphenylalanine and benzene-1,3,5-tricarboxamide (BTA). The cross-β motif is an abundant structural element in amyloid fibrils and aggregates of fibril-forming peptides, including diphenylalanine. The incorporation of topological constraints on one edge of the diphenylalanine fragment limits the number of β-strands in β-sheets and leads to the creation of an unconventional offset patterned cross-β structure consisting of short 3×2 parallel β-sheets stabilized by phenylalanine zippers. In the reported assembly, two patterned cross-β structures bind parallel arrays of BTA stacks in a superstructure within a single-molecule thick nanoribbon. In addition to a threefold network of hydrogen bonds in the BTA stack, each molecule becomes simultaneously bound by hydrogen bonds of three β-sheets and four phenylalanine zippers. The diffuse layer of alkyl chains with terminal polar groups prevents merging of the nanoribbons and stabilizes cross-β-structure in water. Our results provide a simple approach to the incorporation of novel patterned cross-β motifs into supramolecular superstructures and shed light on the general mechanism of β-sheet formation in C3-symmetric peptide amphiphiles.
Snežana Đorđević, María Medel, Justine Hillaert, Esther Masià, Inmaculada Conejos-Sánchez*, María J. Vicent* “Critical Design Strategies Supporting Optimized Drug Release from Polymer-Drug Conjugates” Small. 2023:e2303157. [Small][PubMed]
 The importance of an adequate linking moiety design that allows controlled drug(s) release at the desired site of action has been extensively studied for single and combination polymer-drug conjugates (PDCs) under different pathological scenarios. Redox-responsive self-immolative linkers bearing disulfide moieties (SS-SIL) represent a powerful strategy for intracellular drug delivery; however, the influence of drug structural features and linker-associated spacers on release kinetics remains relatively unexplored. We evaluated the influence of drug/spacer chemical structure and the chemical group available for conjugation on drug release and the biological effect of resultant PDCs. We implemented an artificial intelligence tool (“design of experiments”) to develop a liquid chromatography-mass spectrometry method to perform the exhaustive characterization required for this systematic study. The obtained fit-for-purpose analytical protocol enabled the quantification of low drug concentrations in drug release studies and the elucidation of metabolite presence and provided the first data (to the best of our knowledge) that clarifies how drug structural features influence the drug release from SS-SIL and demonstrates the non-universal nature of the SS-SIL. We highlight the importance of rigorous linker characterization in understanding structure-function correlations between linkers, drug chemical functionalities, and in vitro release kinetics, a critical strategic crafting methodology that should remain under consideration when using a reductive environment as an endogenous drug release trigger.
The importance of an adequate linking moiety design that allows controlled drug(s) release at the desired site of action has been extensively studied for single and combination polymer-drug conjugates (PDCs) under different pathological scenarios. Redox-responsive self-immolative linkers bearing disulfide moieties (SS-SIL) represent a powerful strategy for intracellular drug delivery; however, the influence of drug structural features and linker-associated spacers on release kinetics remains relatively unexplored. We evaluated the influence of drug/spacer chemical structure and the chemical group available for conjugation on drug release and the biological effect of resultant PDCs. We implemented an artificial intelligence tool (“design of experiments”) to develop a liquid chromatography-mass spectrometry method to perform the exhaustive characterization required for this systematic study. The obtained fit-for-purpose analytical protocol enabled the quantification of low drug concentrations in drug release studies and the elucidation of metabolite presence and provided the first data (to the best of our knowledge) that clarifies how drug structural features influence the drug release from SS-SIL and demonstrates the non-universal nature of the SS-SIL. We highlight the importance of rigorous linker characterization in understanding structure-function correlations between linkers, drug chemical functionalities, and in vitro release kinetics, a critical strategic crafting methodology that should remain under consideration when using a reductive environment as an endogenous drug release trigger.
Sonia Vicente-Ruiz, Ana Armiñán*, Katia Maso, Elena Gallon, Oleksandr Zagorodko, Julie Movellan, Fernanda Rodríguez-Otormín, Maike Baues, Jan-Niklas May, Federica De Lorenzi, Twan Lammers, and María J. Vicent* “Poly-L-Glutamic Acid Modification Modulates the Bio-nano Interface of a Therapeutic Anti-IGF-1R Antibody in Prostate Cancer.” Biomaterials, 2023;301:122280 [Biomaterials][PubMed]
 Modifying biological agents with polymers such as polyethylene glycol (PEG) has demonstrated clinical benefits; however, post-market surveillance of PEGylated derivatives has revealed PEG-associated toxicity issues, prompting the search for alternatives. We explore how conjugating a poly-l-glutamic acid (PGA) to an anti-insulin growth factor 1 receptor antibody (AVE1642) modulates the bio-nano interface and anti-tumor activity in preclinical prostate cancer models. Native and PGA-modified AVE1642 display similar anti-tumor activity in vitro; however, AVE1642 prompts IGF-1R internalization while PGA conjugation prompts higher affinity IGF-1R binding, thereby inhibiting IGF-1R internalization and altering cell trafficking. AVE1642 attenuates phosphoinositide 3-kinase signaling, while PGA-AVE1642 inhibits phosphoinositide 3-kinase and mitogen-activated protein kinase signaling. PGA conjugation also enhances AVE1642’s anti-tumor activity in an orthotopic prostate cancer mouse model, while PGA-AVE1642 induces more significant suppression of cancer cell proliferation/angiogenesis than AVE1642. These findings demonstrate that PGA conjugation modulates an antibody’s bio-nano interface, mechanism of action, and therapeutic activity.
Modifying biological agents with polymers such as polyethylene glycol (PEG) has demonstrated clinical benefits; however, post-market surveillance of PEGylated derivatives has revealed PEG-associated toxicity issues, prompting the search for alternatives. We explore how conjugating a poly-l-glutamic acid (PGA) to an anti-insulin growth factor 1 receptor antibody (AVE1642) modulates the bio-nano interface and anti-tumor activity in preclinical prostate cancer models. Native and PGA-modified AVE1642 display similar anti-tumor activity in vitro; however, AVE1642 prompts IGF-1R internalization while PGA conjugation prompts higher affinity IGF-1R binding, thereby inhibiting IGF-1R internalization and altering cell trafficking. AVE1642 attenuates phosphoinositide 3-kinase signaling, while PGA-AVE1642 inhibits phosphoinositide 3-kinase and mitogen-activated protein kinase signaling. PGA conjugation also enhances AVE1642’s anti-tumor activity in an orthotopic prostate cancer mouse model, while PGA-AVE1642 induces more significant suppression of cancer cell proliferation/angiogenesis than AVE1642. These findings demonstrate that PGA conjugation modulates an antibody’s bio-nano interface, mechanism of action, and therapeutic activity.
López-Guerrero, J. A., Valés-Gómez, M., Borrás, F. E., Falcón-Pérez, J. M., Vicent, M. J., & Yáñez-Mó, M. Standardising the preanalytical reporting of biospecimens to improve reproducibility in extracellular vesicle research – A GEIVEX study. Journal of Extracellular Biology, 2023, 2, e76. [Journal of Extracellular Biology]
 The standardization of clinical studies using extracellular vesicles (EVs) has mainly focused on the procedures employed for isolation and characterization; however, preanalytical aspects of sample collection, handling, and storage significantly impact the reproducibility of results. We conducted an online survey based on SPREC (Standard PREanalytical Code) among members of GEIVEX (Grupo Español de Investigacion en Vesiculas Extracelulares) to explore how distinct laboratories handled fluid biospecimens destined for EV analyses. We received 70 surveys from 43 different laboratories: 44% focused on plasma, 9% on serum, and 16% on urine. The survey indicated that variability in preanalytical approaches reaches 94%. In some cases, researchers did not have access to all relevant preanalytical details of the samples, with some sample aspects with potential impact on EV isolation/characterization not coded within the current version of SPREC. Our study highlights the importance of working with common standard operating procedures (SOP) to control preanalytical conditions. The application of SPREC represents a suitable approach to codify and register preanalytical conditions. Integrating SPREC into the SOPs of laboratories/biobanks will provide a valuable source of information and constitute an advance for EV research by improving reproducibility and credibility.
The standardization of clinical studies using extracellular vesicles (EVs) has mainly focused on the procedures employed for isolation and characterization; however, preanalytical aspects of sample collection, handling, and storage significantly impact the reproducibility of results. We conducted an online survey based on SPREC (Standard PREanalytical Code) among members of GEIVEX (Grupo Español de Investigacion en Vesiculas Extracelulares) to explore how distinct laboratories handled fluid biospecimens destined for EV analyses. We received 70 surveys from 43 different laboratories: 44% focused on plasma, 9% on serum, and 16% on urine. The survey indicated that variability in preanalytical approaches reaches 94%. In some cases, researchers did not have access to all relevant preanalytical details of the samples, with some sample aspects with potential impact on EV isolation/characterization not coded within the current version of SPREC. Our study highlights the importance of working with common standard operating procedures (SOP) to control preanalytical conditions. The application of SPREC represents a suitable approach to codify and register preanalytical conditions. Integrating SPREC into the SOPs of laboratories/biobanks will provide a valuable source of information and constitute an advance for EV research by improving reproducibility and credibility.
Tetiana Melnyk, Esther Masiá, Oleksandr Zagorodko, Inmaculada Conejos-Sánchez, and María J. Vicent. “Rational Design of Poly-L-glutamic acid-Palbociclib Conjugates for Pediatric Glioma Treatment.” Journal of Controlled Release. 2023 Mar;355:385-394 [PubMed][J Control Rel]
 Brain tumors represent the second most common cause of pediatric cancer death, with malignant gliomas accounting for ~75% of pediatric deaths. Palbociclib, a selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, has shown promise in phase I clinical trials of pediatric patients with progressive/refractory brain tumors using the oral administration route; however, pharmacokinetic limitations and toxicity issues remain. We synthesized a family of well-defined linear and star-shaped polyglutamate (PGA)-palbociclib conjugates using redox-sensitive self-immolative linkers to overcome limitations associated with free palbociclib. Exhaustive characterization of this conjugate family provided evidence for a transition towards the formation of more organized conformational structures upon increased drug loading. We evaluated the activity of conjugates in patient-derived glioblastoma and diffuse intrinsic pontine glioma cells, which display differing reducing environments due to differential glutathione expression levels. We discovered that microenvironmental parameters and the identified conformational changes determined palbociclib release kinetics and therapeutic output; furthermore, we identified a star-shaped PGA-palbociclib conjugate with low drug loading as an optimal therapeutic approach in diffuse intrinsic pontine glioma cells.
Brain tumors represent the second most common cause of pediatric cancer death, with malignant gliomas accounting for ~75% of pediatric deaths. Palbociclib, a selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, has shown promise in phase I clinical trials of pediatric patients with progressive/refractory brain tumors using the oral administration route; however, pharmacokinetic limitations and toxicity issues remain. We synthesized a family of well-defined linear and star-shaped polyglutamate (PGA)-palbociclib conjugates using redox-sensitive self-immolative linkers to overcome limitations associated with free palbociclib. Exhaustive characterization of this conjugate family provided evidence for a transition towards the formation of more organized conformational structures upon increased drug loading. We evaluated the activity of conjugates in patient-derived glioblastoma and diffuse intrinsic pontine glioma cells, which display differing reducing environments due to differential glutathione expression levels. We discovered that microenvironmental parameters and the identified conformational changes determined palbociclib release kinetics and therapeutic output; furthermore, we identified a star-shaped PGA-palbociclib conjugate with low drug loading as an optimal therapeutic approach in diffuse intrinsic pontine glioma cells.
Zoraida Andreu, Esther Masiá, David Charbonnier, María J. Vicent. “A Rapid, Convergent Approach to the Identification of Exosome Inhibitors in Breast Cancer Models.” Nanotheranostics 2023; 7(1):1-21. [Nanotheranostics]
 Targeting cancer cell exosome release and biogenesis represents a potentially efficient means to treat tumors and prevent cancer recurrence/metastasis; however, the complexity and time-consuming nature of currently employed methods to purify and characterize exosomes represent obstacles to progression. Herein, we describe a rapid, convergent, and cost-efficient strategy to analyze candidate FDA-approved drugs that inhibit exosome release and/or biogenesis using breast cancer cell line models in the hope of repurposing them for the clinical treatment of metastatic tumors. We combined the ExoScreen assay based on AlphaScreenTM technology with the antibody-mediated detection of an atypical lipid (lysobisphosphatidic acid – LBPA) present in the intra-luminal vesicle/exosomal fraction to achieve both extracellular and intracellular information on exosome modulation after treatment. As proof of concept for this strategy, we identified MDA-MB-453 in the Her-2 positive cell line and docetaxel, biscurcumin, primaquine, and doxorubicin in the luminal A MCF7 cell line as potential exosome release inhibitors. Dinaciclib also functioned as an exosome release inhibitor in MCF7 cells. Further, we explored the expression of proteins involved in exosome biogenesis (TSG101, CD9 tetraspanin, Alix, SMase2) and release (Rab11, Rab27) to decipher and validate the possible molecular mechanisms of action of the identified exosome inhibitors. We anticipate that our approach could help to create robust high-throughput screening methodologies to accelerate drug repurposing when using FDA-approved compound libraries and to develop rationally-designed single/combination therapies such as nanomedicines that can target metastasis progression by modulating exosome biogenesis or release in various tumor types.
Targeting cancer cell exosome release and biogenesis represents a potentially efficient means to treat tumors and prevent cancer recurrence/metastasis; however, the complexity and time-consuming nature of currently employed methods to purify and characterize exosomes represent obstacles to progression. Herein, we describe a rapid, convergent, and cost-efficient strategy to analyze candidate FDA-approved drugs that inhibit exosome release and/or biogenesis using breast cancer cell line models in the hope of repurposing them for the clinical treatment of metastatic tumors. We combined the ExoScreen assay based on AlphaScreenTM technology with the antibody-mediated detection of an atypical lipid (lysobisphosphatidic acid – LBPA) present in the intra-luminal vesicle/exosomal fraction to achieve both extracellular and intracellular information on exosome modulation after treatment. As proof of concept for this strategy, we identified MDA-MB-453 in the Her-2 positive cell line and docetaxel, biscurcumin, primaquine, and doxorubicin in the luminal A MCF7 cell line as potential exosome release inhibitors. Dinaciclib also functioned as an exosome release inhibitor in MCF7 cells. Further, we explored the expression of proteins involved in exosome biogenesis (TSG101, CD9 tetraspanin, Alix, SMase2) and release (Rab11, Rab27) to decipher and validate the possible molecular mechanisms of action of the identified exosome inhibitors. We anticipate that our approach could help to create robust high-throughput screening methodologies to accelerate drug repurposing when using FDA-approved compound libraries and to develop rationally-designed single/combination therapies such as nanomedicines that can target metastasis progression by modulating exosome biogenesis or release in various tumor types.
Esther Giraldo, Pablo Bonilla, Mara Mellado, Pablo Garcia-Manau, Carlota Rodo, Ana Alastrue, Eric Lopez, Elena Carreras Moratonas, Ferran Pellise, Snežana Đorđević, María J. Vicent, and Victoria Moreno Manzano. “Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury.” Cells. 2022, 11(20), 3304. [Cells][PubMed]
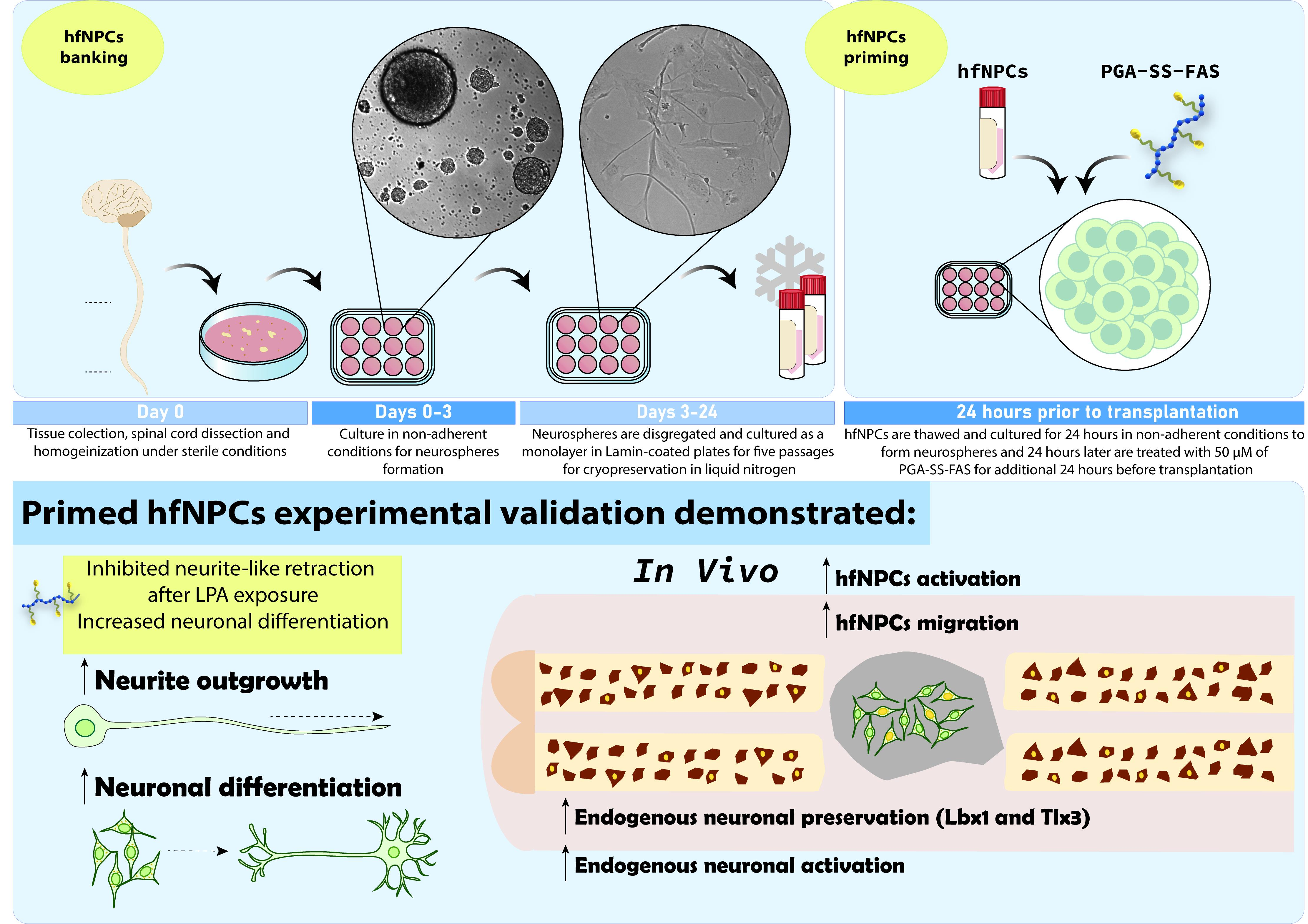 Neural precursor cell (NPC) transplantation represents a promising therapy for treating spinal cord injuries (SCIs); however, despite successful results obtained in preclinical models, the clinical translation of this approach remains challenging due, in part, to the lack of consensus on an optimal cell source for human neuronal cells. Depending on the cell source, additional limitations to NPC-based therapies include high tumorigenic potential, alongside poor graft survival and engraftment into host spinal tissue. We previously demonstrated that NPCs derived from rat fetal spinal cords primed with a polyglutamate (PGA)-conjugated form of the Rho/Rock inhibitor fasudil (PGA-SS-FAS) displayed enhanced neuronal differentiation and graft survival when compared to non-primed NPCs. We now conducted a similar study of human-fetal-spinal-cord-derived NPCs (hfNPCs) from legal gestational interruptions at the late gestational stage, at 19–21.6 weeks. In vitro, expanded hfNPCs retained neural features, multipotency, and self-renewal, which supported the development of a cell banking strategy. Before transplantation, we established a simple procedure to prime hfNPCs by overnight incubation with PGA-SS-FAS (at 50 μM FAS equiv.), which improved neuronal differentiation and overcame neurite-like retraction after lysophosphatidic-acid-induced Rho/Rock activation. The transplantation of primed hfNPCs into immune-deficient mice (NU(NCr)-Foxn1nu) immediately after the eighth thoracic segment compression prompted enhanced migration of grafted cells from the dorsal to the ventral spinal cord, increased preservation of GABAergic inhibitory Lbx1-expressing and glutamatergic excitatory Tlx3-expressing somatosensory interneurons, and elevated the numbers of preserved, c-Fos-expressing, activated neurons surrounding the injury epicenter, all in a low percentage. Overall, the priming procedure using PGA-SS-FAS could represent an alternative methodology to improve the capabilities of the hfNPC lines for a translational approach for acute SCI treatment.
Neural precursor cell (NPC) transplantation represents a promising therapy for treating spinal cord injuries (SCIs); however, despite successful results obtained in preclinical models, the clinical translation of this approach remains challenging due, in part, to the lack of consensus on an optimal cell source for human neuronal cells. Depending on the cell source, additional limitations to NPC-based therapies include high tumorigenic potential, alongside poor graft survival and engraftment into host spinal tissue. We previously demonstrated that NPCs derived from rat fetal spinal cords primed with a polyglutamate (PGA)-conjugated form of the Rho/Rock inhibitor fasudil (PGA-SS-FAS) displayed enhanced neuronal differentiation and graft survival when compared to non-primed NPCs. We now conducted a similar study of human-fetal-spinal-cord-derived NPCs (hfNPCs) from legal gestational interruptions at the late gestational stage, at 19–21.6 weeks. In vitro, expanded hfNPCs retained neural features, multipotency, and self-renewal, which supported the development of a cell banking strategy. Before transplantation, we established a simple procedure to prime hfNPCs by overnight incubation with PGA-SS-FAS (at 50 μM FAS equiv.), which improved neuronal differentiation and overcame neurite-like retraction after lysophosphatidic-acid-induced Rho/Rock activation. The transplantation of primed hfNPCs into immune-deficient mice (NU(NCr)-Foxn1nu) immediately after the eighth thoracic segment compression prompted enhanced migration of grafted cells from the dorsal to the ventral spinal cord, increased preservation of GABAergic inhibitory Lbx1-expressing and glutamatergic excitatory Tlx3-expressing somatosensory interneurons, and elevated the numbers of preserved, c-Fos-expressing, activated neurons surrounding the injury epicenter, all in a low percentage. Overall, the priming procedure using PGA-SS-FAS could represent an alternative methodology to improve the capabilities of the hfNPC lines for a translational approach for acute SCI treatment.
Anni Lepland, Alessio Malfanti, Uku Haljasorg, Eliana Asciutto, Monica Pickholz, Mauro Bringas, Snežana Đorđević, Liis Salumäe, Pärt Peterson, Tambet Teesalu, María J. Vicent, Pablo Scodeller. “Depletion of CD206+ Tumour Macrophages via a Peptide-Targeted Star-Shaped Polyglutamate Inhibits Tumourigenesis and Metastatic Dissemination in Breast Cancer in Mice.” Cancer Research Communications. 2022, 2(6), 533–551. [Cancer Research Communications]
 Although many studies have explored the depletion of tumour-associated macrophages (TAMs) as a therapeutic strategy for solid tumours, currently available compounds suffer from poor efficacy and dose-limiting side effects. Here, we developed a novel TAM-depleting agent (“OximUNO”) that specifically targets CD206+ TAMs and demonstrated efficacy in triple negative breast cancer (TNBC) mouse models. OximUNO comprises a star-shaped polyglutamate (St-PGA) decorated with the CD206-targeting peptide mUNO that carries the chemotherapeutic drug doxorubicin (DOX). In TNBC models, a fluorescently-labelled mUNO-decorated St-PGA homed to CD206+ TAMs within primary lesions and metastases. OximUNO exhibited no acute liver or kidney toxicity in vivo. Treatment with OximUNO reduced the progression of primary tumour lesions and pulmonary metastases, significantly diminished the number of CD206+ TAMs and increased the CD8/FOXP3 expression ratio (demonstrating immunostimulation). Our findings suggest the potential benefit of OximUNO as a TAM-depleting agent for TNBC treatment. Importantly, our studies also represent the first report of a peptide-targeted St-PGA as a targeted therapeutic nanoconjugate.
Although many studies have explored the depletion of tumour-associated macrophages (TAMs) as a therapeutic strategy for solid tumours, currently available compounds suffer from poor efficacy and dose-limiting side effects. Here, we developed a novel TAM-depleting agent (“OximUNO”) that specifically targets CD206+ TAMs and demonstrated efficacy in triple negative breast cancer (TNBC) mouse models. OximUNO comprises a star-shaped polyglutamate (St-PGA) decorated with the CD206-targeting peptide mUNO that carries the chemotherapeutic drug doxorubicin (DOX). In TNBC models, a fluorescently-labelled mUNO-decorated St-PGA homed to CD206+ TAMs within primary lesions and metastases. OximUNO exhibited no acute liver or kidney toxicity in vivo. Treatment with OximUNO reduced the progression of primary tumour lesions and pulmonary metastases, significantly diminished the number of CD206+ TAMs and increased the CD8/FOXP3 expression ratio (demonstrating immunostimulation). Our findings suggest the potential benefit of OximUNO as a TAM-depleting agent for TNBC treatment. Importantly, our studies also represent the first report of a peptide-targeted St-PGA as a targeted therapeutic nanoconjugate.
Hoda Elkhenany, Pablo Bonilla, Esther Giraldo, Ana Alastrue Agudo, Michael Edel, María Jesus Vicent, Fernando Gisbert Roca, Laura Rodríguez Doblado, Cristina Martínez-Ramos, Manuel Monleón Pradas, Victoria Moreno-Manzano. “A Hyaluronic Acid Demilune Scaffold and Polypyrrole-coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries”. Biomedicines. 2021, 9(12), 1928. [Biomedicines][PubMed]
 Tissue engineering, including cell transplantation and the application of biomaterials and bioactive molecules, represents a promising approach for regeneration following spinal cord injury (SCI). We designed a combinatorial tissue-engineered approach for the minimally invasive treatment of SCI—a hyaluronic acid (HA)-based scaffold containing polypyrrole-coated fibers (PPY) combined with the RAD16-I self-assembling peptide hydrogel (Corning® PuraMatrix™ peptide hydrogel (PM)), human induced neural progenitor cells (iNPCs), and a nanoconjugated form of curcumin (CURC). In vitro cultures demonstrated that PM preserves iNPC viability and the addition of CURC reduces apoptosis and enhances the outgrowth of Nestin-positive neurites from iNPCs, compared to non-embedded iNPCs. The treatment of spinal cord organotypic cultures also demonstrated that CURC enhances cell migration and prompts a neuron-like morphology of embedded iNPCs implanted over the tissue slices. Following sub-acute SCI by traumatic contusion in rats, the implantation of PM-embedded iNPCs and CURC with PPY fibers supported a significant increase in neuro-preservation (as measured by greater βIII-tubulin staining of neuronal fibers) and decrease in the injured area (as measured by the lack of GFAP staining). This combination therapy also restricted platelet-derived growth factor expression, indicating a reduction in fibrotic pericyte invasion. Overall, these findings support PM-embedded iNPCs with CURC placed within an HA demilune scaffold containing PPY fibers as a minimally invasive combination-based alternative to cell transplantation alone.
Tissue engineering, including cell transplantation and the application of biomaterials and bioactive molecules, represents a promising approach for regeneration following spinal cord injury (SCI). We designed a combinatorial tissue-engineered approach for the minimally invasive treatment of SCI—a hyaluronic acid (HA)-based scaffold containing polypyrrole-coated fibers (PPY) combined with the RAD16-I self-assembling peptide hydrogel (Corning® PuraMatrix™ peptide hydrogel (PM)), human induced neural progenitor cells (iNPCs), and a nanoconjugated form of curcumin (CURC). In vitro cultures demonstrated that PM preserves iNPC viability and the addition of CURC reduces apoptosis and enhances the outgrowth of Nestin-positive neurites from iNPCs, compared to non-embedded iNPCs. The treatment of spinal cord organotypic cultures also demonstrated that CURC enhances cell migration and prompts a neuron-like morphology of embedded iNPCs implanted over the tissue slices. Following sub-acute SCI by traumatic contusion in rats, the implantation of PM-embedded iNPCs and CURC with PPY fibers supported a significant increase in neuro-preservation (as measured by greater βIII-tubulin staining of neuronal fibers) and decrease in the injured area (as measured by the lack of GFAP staining). This combination therapy also restricted platelet-derived growth factor expression, indicating a reduction in fibrotic pericyte invasion. Overall, these findings support PM-embedded iNPCs with CURC placed within an HA demilune scaffold containing PPY fibers as a minimally invasive combination-based alternative to cell transplantation alone.
Paula M Soriano-Teruel, Guillermo García-Laínez, María Marco-Salvador, Julian Pardo, Maykel Arias, Christian De Ford, Irmgard Merfort, María J Vicent, Pablo Pelegrin, Monica Sancho, and Mar Orzáez. “Identification of an ASC Oligomerization Inhibitor for the Treatment of Inflammatory Diseases.” Cell Death & Disease. 2021, 12, 1155 [Journal Website][PubMed]
 The ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD)) protein is an scaffold component of different inflammasomes, intracellular multiprotein platforms of the innate immune system that are activated in response to pathogens or intracellular damage. The formation of ASC specks, initiated by different inflammasome receptors, promotes the recruitment and activation of procaspase-1, thereby triggering pyroptotic inflammatory cell death and pro-inflammatory cytokine release. Here we describe MM01 as the first-in-class small-molecule inhibitor of ASC that interferes with ASC speck formation. MM01 inhibition of ASC oligomerization prevents activation of procaspase-1 in vitro and inhibits the activation of different ASC-dependent inflammasomes in cell lines and primary cultures. Furthermore, MM01 inhibits inflammation in vivo in a mouse model of inflammasome-induced peritonitis. Overall, we highlight MM01 as a novel broad-spectrum inflammasome inhibitor for the potential treatment of multifactorial diseases involving the dysregulation of multiple inflammasomes.
The ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD)) protein is an scaffold component of different inflammasomes, intracellular multiprotein platforms of the innate immune system that are activated in response to pathogens or intracellular damage. The formation of ASC specks, initiated by different inflammasome receptors, promotes the recruitment and activation of procaspase-1, thereby triggering pyroptotic inflammatory cell death and pro-inflammatory cytokine release. Here we describe MM01 as the first-in-class small-molecule inhibitor of ASC that interferes with ASC speck formation. MM01 inhibition of ASC oligomerization prevents activation of procaspase-1 in vitro and inhibits the activation of different ASC-dependent inflammasomes in cell lines and primary cultures. Furthermore, MM01 inhibits inflammation in vivo in a mouse model of inflammasome-induced peritonitis. Overall, we highlight MM01 as a novel broad-spectrum inflammasome inhibitor for the potential treatment of multifactorial diseases involving the dysregulation of multiple inflammasomes.
Sanz, F. J., C. Solana-Manrique, J. Torres, E. Masiá, M. J. Vicent and N. Paricio. “A High-Throughput Chemical Screen in DJ-1β Mutant Flies Identifies Zaprinast as a Potential Parkinson’s Disease Treatment.” Neurotherapeutics. 2021, 18(4):2565-2578 [PubMed][Journal Website]
 Dopamine replacement represents the standard therapy for Parkinson’s disease (PD), a common, chronic, and incurable neurological disorder; however, this approach only treats the symptoms of this devastating disease. In the search for novel disease-modifying therapies that target other relevant molecular and cellular mechanisms, Drosophila has emerged as a valuable tool to study neurodegenerative diseases due to the presence of a complex central nervous system, the blood–brain barrier, and a similar neurotransmitter profile to humans. Human PD-related genes also display conservation in flies; DJ-1β is the fly ortholog of DJ-1, a gene for which mutations prompt early-onset recessive PD. Interestingly, flies mutant for DJ-1β exhibit PD-related phenotypes, including motor defects, high oxidative stress (OS) levels and metabolic alterations. To identify novel therapies for PD, we performed an in vivo high-throughput screening assay using DJ-1β mutant flies and compounds from the Prestwick® chemical library. Drugs that improved motor performance in DJ-1ß mutant flies were validated in DJ-1-deficient human neural-like cells, revealing that zaprinast displayed the most significant ability to suppress OS-induced cell death. Zaprinast inhibits phosphodiesterases and activates GPR35, an orphan G-protein-coupled receptor not previously associated with PD. We found that zaprinast exerts its beneficial effect in both fly and human PD models through several disease-modifying mechanisms, including reduced OS levels, attenuated apoptosis, increased mitochondrial viability, and enhanced glycolysis. Therefore, our results support zaprinast as a potential therapeutic for PD in future clinical trials.
Dopamine replacement represents the standard therapy for Parkinson’s disease (PD), a common, chronic, and incurable neurological disorder; however, this approach only treats the symptoms of this devastating disease. In the search for novel disease-modifying therapies that target other relevant molecular and cellular mechanisms, Drosophila has emerged as a valuable tool to study neurodegenerative diseases due to the presence of a complex central nervous system, the blood–brain barrier, and a similar neurotransmitter profile to humans. Human PD-related genes also display conservation in flies; DJ-1β is the fly ortholog of DJ-1, a gene for which mutations prompt early-onset recessive PD. Interestingly, flies mutant for DJ-1β exhibit PD-related phenotypes, including motor defects, high oxidative stress (OS) levels and metabolic alterations. To identify novel therapies for PD, we performed an in vivo high-throughput screening assay using DJ-1β mutant flies and compounds from the Prestwick® chemical library. Drugs that improved motor performance in DJ-1ß mutant flies were validated in DJ-1-deficient human neural-like cells, revealing that zaprinast displayed the most significant ability to suppress OS-induced cell death. Zaprinast inhibits phosphodiesterases and activates GPR35, an orphan G-protein-coupled receptor not previously associated with PD. We found that zaprinast exerts its beneficial effect in both fly and human PD models through several disease-modifying mechanisms, including reduced OS levels, attenuated apoptosis, increased mitochondrial viability, and enhanced glycolysis. Therefore, our results support zaprinast as a potential therapeutic for PD in future clinical trials.
Yolanda Fernández, Julie Movellan, Laia Foradada, Vanessa Giménez, Natalia García-Aranda, Sandra Mancilla, Ana Armiñán, Sven Even Borgos, Astrid Hyldbakk, Anna Bogdansk, Oliviero L. Gobbo, Adriele Prina-Mello, Jessica Ponti, Luigi Calzolai, Oleksandr Zagorodko, Elena Gallon, Amaya Niño-Pariente, Alison Paul, Simó Schwartz Jr., Ibane Abasolo*, María J. Vicent.* “In Vivo Antitumor and Antimetastatic Efficacy of a Polyacetal-Based Paclitaxel Conjugate for Prostate Cancer Therapy” Advanced Healthcare Materials. 2021; e2101544. [PubMed][Journal Website]
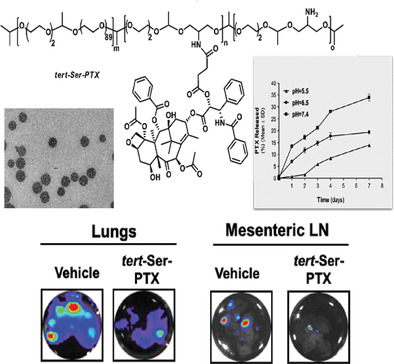 Prostate cancer (PCa), one of the leading causes of cancer-related deaths, currently lacks effective treatment for advanced-stage disease. Paclitaxel (PTX) is a highly active chemotherapeutic drug and the first-line treatment for PCa; however, conventional PTX formulation causes severe hypersensitivity reactions and limits PTX use at high concentrations. In the pursuit of high molecular weight, biodegradable, and pH-responsive polymeric carriers, we conjugated PTX to a polyacetal-based nanocarrier to yield a tert-Ser-PTX polyacetal conjugate. tert-Ser-PTX conjugate provides sustained release of PTX over two weeks in a pH-responsive manner while also obtaining a degree of epimerization of PTX to 7-epi-PTX. Serum proteins stabilize tert-Ser-PTX, with enhanced stability in human serum vs. PBS (pH 7.4). In vitro efficacy assessments in PCa cells demonstrated IC50 values above those for the free form of PTX due to the differential cell trafficking modes; however, in vivo tolerability assays demonstrated that tert-Ser-PTX significantly reduced the systemic toxicities associated with free PTX treatment. tert-Ser-PTX also effectively inhibited primary tumor growth and hematologic, lymphatic, and coelomic dissemination, as confirmed by in vivo and ex vivo bioluminescence imaging and histopathological evaluations in mice carrying orthotopic LNCaP tumors. Overall, our results suggest the application of tert-Ser-PTX as a robust anti-tumor/antimetastatic treatment for PCa.
Prostate cancer (PCa), one of the leading causes of cancer-related deaths, currently lacks effective treatment for advanced-stage disease. Paclitaxel (PTX) is a highly active chemotherapeutic drug and the first-line treatment for PCa; however, conventional PTX formulation causes severe hypersensitivity reactions and limits PTX use at high concentrations. In the pursuit of high molecular weight, biodegradable, and pH-responsive polymeric carriers, we conjugated PTX to a polyacetal-based nanocarrier to yield a tert-Ser-PTX polyacetal conjugate. tert-Ser-PTX conjugate provides sustained release of PTX over two weeks in a pH-responsive manner while also obtaining a degree of epimerization of PTX to 7-epi-PTX. Serum proteins stabilize tert-Ser-PTX, with enhanced stability in human serum vs. PBS (pH 7.4). In vitro efficacy assessments in PCa cells demonstrated IC50 values above those for the free form of PTX due to the differential cell trafficking modes; however, in vivo tolerability assays demonstrated that tert-Ser-PTX significantly reduced the systemic toxicities associated with free PTX treatment. tert-Ser-PTX also effectively inhibited primary tumor growth and hematologic, lymphatic, and coelomic dissemination, as confirmed by in vivo and ex vivo bioluminescence imaging and histopathological evaluations in mice carrying orthotopic LNCaP tumors. Overall, our results suggest the application of tert-Ser-PTX as a robust anti-tumor/antimetastatic treatment for PCa.
Giraldo, E., V. J. Nebot, S. Đorđević, R. Requejo-Aguilar, A. Alastrue-Agudo, O. Zagorodko, A. Armiñan, B. Martinez-Rojas, M. J. Vicent* and V. Moreno-Manzano*. “A rationally designed self-immolative linker enhances the synergism between a polymer-rock inhibitor conjugate and neural progenitor cells in the treatment of spinal cord injury.” Biomaterials. 2021; 276: 121052. [Biomaterials][PubMed]
 Rho/ROCK signaling induced after spinal cord injury (SCI) contributes to secondary damage by promoting apoptosis, inflammation, and axon growth inhibition. The specific Rho-kinase inhibitor fasudil can contribute to functional regeneration after SCI, although inherent low stability has hampered its use. To improve the therapeutic potential of fasudil, we now describe a family of rationally-designed bioresponsive polymer-fasudil conjugates based on an understanding of the conditions after SCI, such as low pH, enhanced expression of specific proteases, and a reductive environment. Fasudil conjugated to poly-l-glutamate via a self-immolative redox-sensitive linker (PGA-SS-F) displays optimal release kinetics and, consequently, treatment with PGA-SS-F significantly induces neurite elongation and axon growth in dorsal root ganglia explants, spinal cord organotypic cultures, and neural precursor cells (NPCs). The intrathecal administration of PGA-SS-F after SCI in a rat model prevents early apoptosis and induces the expression of axonal growth- and neuroplasticity-associated markers to a higher extent than the free form of fasudil. Moreover, a combination treatment comprising the acute transplantation of NPCs pre-treated with PGA-SS-F leads to enhanced cell engraftment and reduced cyst formation after SCI. In chronic SCI, combinatory treatment increases the preservation of neuronal fibers. Overall, this synergistic combinatorial strategy may represent a potentially efficient clinical approach to SCI treatment.
Rho/ROCK signaling induced after spinal cord injury (SCI) contributes to secondary damage by promoting apoptosis, inflammation, and axon growth inhibition. The specific Rho-kinase inhibitor fasudil can contribute to functional regeneration after SCI, although inherent low stability has hampered its use. To improve the therapeutic potential of fasudil, we now describe a family of rationally-designed bioresponsive polymer-fasudil conjugates based on an understanding of the conditions after SCI, such as low pH, enhanced expression of specific proteases, and a reductive environment. Fasudil conjugated to poly-l-glutamate via a self-immolative redox-sensitive linker (PGA-SS-F) displays optimal release kinetics and, consequently, treatment with PGA-SS-F significantly induces neurite elongation and axon growth in dorsal root ganglia explants, spinal cord organotypic cultures, and neural precursor cells (NPCs). The intrathecal administration of PGA-SS-F after SCI in a rat model prevents early apoptosis and induces the expression of axonal growth- and neuroplasticity-associated markers to a higher extent than the free form of fasudil. Moreover, a combination treatment comprising the acute transplantation of NPCs pre-treated with PGA-SS-F leads to enhanced cell engraftment and reduced cyst formation after SCI. In chronic SCI, combinatory treatment increases the preservation of neuronal fibers. Overall, this synergistic combinatorial strategy may represent a potentially efficient clinical approach to SCI treatment.
Bonilla P, Hernandez J, Giraldo E, González-Pérez MA, Alastrue-Agudo A, Elkhenany H, Vicent MJ, Navarro X, Edel M, Moreno-Manzano V*. “Human-Induced Neural and Mesenchymal Stem Cell Therapy Combined with a Curcumin Nanoconjugate as a Spinal Cord Injury Treatment.” International Journal of Molecular Sciences. 2021; 22(11):5966. [Free Download at Int .J. Mol. Sci.][PubMed]
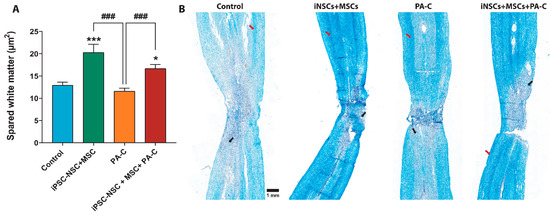 We currently lack effective treatments for the devastating loss of neural function associated with spinal cord injury (SCI). In this study, we evaluated a combination therapy comprising human neural stem cells derived from induced pluripotent stem cells (iPSC-NSC), human mesenchymal stem cells (MSC), and a pH-responsive polyacetal–curcumin nanoconjugate (PA-C) that allows the sustained release of curcumin. In vitro analysis demonstrated that PA-C treatment protected iPSC-NSC from oxidative damage in vitro, while MSC co-culture prevented lipopolysaccharide-induced activation of nuclear factor-κB (NF-κB) in iPSC-NSC. Then, we evaluated the combination of PA-C delivery into the intrathecal space in a rat model of contusive SCI with stem cell transplantation. While we failed to observe significant improvements in locomotor function (BBB scale) in treated animals, histological analysis revealed that PA-C-treated or PA-C and iPSC-NSC + MSC-treated animals displayed significantly smaller scars, while PA-C and iPSC-NSC + MSC treatment induced the preservation of β-III Tubulin-positive axons. iPSC-NSC + MSC transplantation fostered the preservation of motoneurons and myelinated tracts, while PA-C treatment polarized microglia into an anti-inflammatory phenotype. Overall, the combination of stem cell transplantation and PA-C treatment confers higher neuroprotective effects compared to individual treatments.
We currently lack effective treatments for the devastating loss of neural function associated with spinal cord injury (SCI). In this study, we evaluated a combination therapy comprising human neural stem cells derived from induced pluripotent stem cells (iPSC-NSC), human mesenchymal stem cells (MSC), and a pH-responsive polyacetal–curcumin nanoconjugate (PA-C) that allows the sustained release of curcumin. In vitro analysis demonstrated that PA-C treatment protected iPSC-NSC from oxidative damage in vitro, while MSC co-culture prevented lipopolysaccharide-induced activation of nuclear factor-κB (NF-κB) in iPSC-NSC. Then, we evaluated the combination of PA-C delivery into the intrathecal space in a rat model of contusive SCI with stem cell transplantation. While we failed to observe significant improvements in locomotor function (BBB scale) in treated animals, histological analysis revealed that PA-C-treated or PA-C and iPSC-NSC + MSC-treated animals displayed significantly smaller scars, while PA-C and iPSC-NSC + MSC treatment induced the preservation of β-III Tubulin-positive axons. iPSC-NSC + MSC transplantation fostered the preservation of motoneurons and myelinated tracts, while PA-C treatment polarized microglia into an anti-inflammatory phenotype. Overall, the combination of stem cell transplantation and PA-C treatment confers higher neuroprotective effects compared to individual treatments.O. Zagorodko, T. Melnyk, O. Rogier, V.J. Nebot, M.J. Vicent*, Higher-order interfiber interactions in the self-assembly of benzene-1,3,5-tricarboxamide-based peptides in water. Polymer Chemistry. 2021;12:3478-3487 [Free Download at Polymer Chemistry][Zenodo][PubMed]
 Mimicking the complexity of biological systems with synthetic supramolecular materials requires a deep understanding of the relationship between the structure of the molecule and its self-assembly pattern. Herein, we report a series of water-soluble benzene-1,3,5-tricarboxamide-based di- and tripeptide derivatives modified with small non-bulky terminal amine salt to induce self-assembly into twisted one-dimensional higher-order nanofibers. The morphology of nanofibers strongly depends on the nature, order, and quantity of amino acids in the short peptide fragments and vary from simple cylindrical to complex helical. From observations of several fiber-splitting events, we detected interfiber interactions that always occur in a pairwise manner, which implies that the C3 symmetry of benzene-1,3,5-tricarboxamide-based molecules in higher-order fibers becomes gradually distorted, thus facilitating hydrophobic contact interactions between fibrils. The proposed mechanism of self-assembly through hydrophobic contact allowed the successful design of a compound with pH-responsive morphology, and may find use in the future development of complex hierarchical architectures with controlled functionality.
Mimicking the complexity of biological systems with synthetic supramolecular materials requires a deep understanding of the relationship between the structure of the molecule and its self-assembly pattern. Herein, we report a series of water-soluble benzene-1,3,5-tricarboxamide-based di- and tripeptide derivatives modified with small non-bulky terminal amine salt to induce self-assembly into twisted one-dimensional higher-order nanofibers. The morphology of nanofibers strongly depends on the nature, order, and quantity of amino acids in the short peptide fragments and vary from simple cylindrical to complex helical. From observations of several fiber-splitting events, we detected interfiber interactions that always occur in a pairwise manner, which implies that the C3 symmetry of benzene-1,3,5-tricarboxamide-based molecules in higher-order fibers becomes gradually distorted, thus facilitating hydrophobic contact interactions between fibrils. The proposed mechanism of self-assembly through hydrophobic contact allowed the successful design of a compound with pH-responsive morphology, and may find use in the future development of complex hierarchical architectures with controlled functionality.
A. Duro-Castano, C. Borras, V. Herranz-Pérez, M. C. Blanco-Gandía, I. Conejos-Sánchez, A. Armiñán, C. Mas-Bargues, M. Inglés, J. Miñarro, M. Rodríguez-Arias, J. M. García-Verdugo, J. Viña, M. J. Vicent*. Targeting Alzheimer’s disease with multimodal polypeptide-based nanoconjugates. Science Advances 2021;7:eabf9180. [PubMed][Science Advances][Zenodo]
 Alzheimer’s disease (AD), the most prevalent form of dementia, remains incurable mainly due to our failings in the search for effective pharmacological strategies. Here, we describe the development of targeted multimodal polypeptide-based nanoconjugates as potential AD treatments. Treatment with polypeptide nanoconjugates bearing propargylamine moieties and bisdemethoxycurcumin or genistein afforded neuroprotection and displayed neurotrophic effects, as evidenced by an increase in dendritic density of pyramidal neurons in organotypic hippocampal culture. The additional conjugation of the Angiopep-2 targeting moiety enhanced nanoconjugate passage through the blood-brain barrier and modulated brain distribution with nanoconjugate accumulation in neurogenic areas, including the olfactory bulb. Nanoconjugate treatment effectively reduced neurotoxic β amyloid aggregate levels and rescued impairments to olfactory memory and object recognition in APP/PS1 transgenic AD model mice. Overall, this study provides a description of a targeted multimodal polyglutamate-based nanoconjugate with neuroprotective and neurotrophic potential for AD treatment.
Alzheimer’s disease (AD), the most prevalent form of dementia, remains incurable mainly due to our failings in the search for effective pharmacological strategies. Here, we describe the development of targeted multimodal polypeptide-based nanoconjugates as potential AD treatments. Treatment with polypeptide nanoconjugates bearing propargylamine moieties and bisdemethoxycurcumin or genistein afforded neuroprotection and displayed neurotrophic effects, as evidenced by an increase in dendritic density of pyramidal neurons in organotypic hippocampal culture. The additional conjugation of the Angiopep-2 targeting moiety enhanced nanoconjugate passage through the blood-brain barrier and modulated brain distribution with nanoconjugate accumulation in neurogenic areas, including the olfactory bulb. Nanoconjugate treatment effectively reduced neurotoxic β amyloid aggregate levels and rescued impairments to olfactory memory and object recognition in APP/PS1 transgenic AD model mice. Overall, this study provides a description of a targeted multimodal polyglutamate-based nanoconjugate with neuroprotective and neurotrophic potential for AD treatment.
S. Tejedor, I Dolz-Pérez, C G. Decker, A Hernándiz, J L. Diez, R Álvarez, D Castellano, NA. García, I Ontoria-Oviedo, VJ. Nebot, H González-King, B Igual, P Sepúlveda*, M J. Vicent* Polymer Conjugation Potentiates Cardioprotective Therapy in Preclinical Models of Myocardial Ischemia/Reperfusion Injury. Adv. Healthcare Mat. 2021;10:2002121. [Journal Site][PubMed]
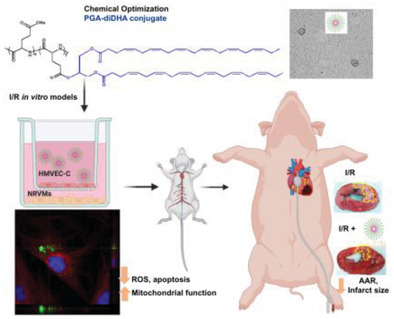 While coronary angioplasty represents an effective treatment option following acute myocardial infarction, the reperfusion of the occluded coronary artery can prompt ischemia–reperfusion (I/R) injury that significantly impacts patient outcomes. As ω‐3 polyunsaturated fatty acids (PUFAs) have proven, yet limited cardioprotective abilities, an optimized polymer‐conjugation approach is reported that improves PUFAs bioavailability to enhance cardioprotection and recovery in animal models of I/R‐induced injury. Poly‐l‐glutamic acid (PGA) conjugation improves the solubility and stability of di‐docosahexaenoic acid (diDHA) under physiological conditions and protects rat neonatal ventricular myocytes from I/R injury by reducing apoptosis, attenuating autophagy, inhibiting reactive oxygen species generation, and restoring mitochondrial membrane potential. Enhanced protective abilities are associated with optimized diDHA loading and evidence is provided for the inherent cardioprotective potential of PGA itself. Pretreatment with PGA–diDHA before reperfusion in a small animal I/R model provides for cardioprotection and limits area at risk (AAR). Furthermore, the preliminary findings suggest that PGA–diDHA administration in a swine I/R model may provide cardioprotection, limit edema and decrease AAR. Overall, the evaluation of PGA–diDHA in relevant preclinical models provides evidence for the potential of polymer‐conjugated PUFAs in the mitigation of I/R injury associated with coronary angioplasty.
While coronary angioplasty represents an effective treatment option following acute myocardial infarction, the reperfusion of the occluded coronary artery can prompt ischemia–reperfusion (I/R) injury that significantly impacts patient outcomes. As ω‐3 polyunsaturated fatty acids (PUFAs) have proven, yet limited cardioprotective abilities, an optimized polymer‐conjugation approach is reported that improves PUFAs bioavailability to enhance cardioprotection and recovery in animal models of I/R‐induced injury. Poly‐l‐glutamic acid (PGA) conjugation improves the solubility and stability of di‐docosahexaenoic acid (diDHA) under physiological conditions and protects rat neonatal ventricular myocytes from I/R injury by reducing apoptosis, attenuating autophagy, inhibiting reactive oxygen species generation, and restoring mitochondrial membrane potential. Enhanced protective abilities are associated with optimized diDHA loading and evidence is provided for the inherent cardioprotective potential of PGA itself. Pretreatment with PGA–diDHA before reperfusion in a small animal I/R model provides for cardioprotection and limits area at risk (AAR). Furthermore, the preliminary findings suggest that PGA–diDHA administration in a swine I/R model may provide cardioprotection, limit edema and decrease AAR. Overall, the evaluation of PGA–diDHA in relevant preclinical models provides evidence for the potential of polymer‐conjugated PUFAs in the mitigation of I/R injury associated with coronary angioplasty.
C.M. Cuesta, F. Ibañez, R. Lopez-Hidalgo, J. Ureña, A. Duro-Castano, A. Armiñán, M.J. Vicent, M. Pascual, C. Guerri. A targeted polypeptide-based Nanoconjugate as a Nanotherapeutic for alcohol-induced Neuroinflammation. Nanomedicine: Nanotechnology, Biology and Medicine. 2021;34:102376. [Journal Site][PubMed]
 Alcohol abuse induces the expression of inflammatory mediators by activating the immune receptors to trigger neuroinflammation and brain damage; however, therapies that reduce neuroimmune system activation may protect against alcohol’s damaging effects. Curcuminoids possess anti-inflammatory properties but suffer from low bioavailability; therefore, we designed a new receptor-targeted biodegradable star-shaped crosslinked polypeptide polymer that bears propargylamine moieties and bisdemethoxycurcumin (StClPr-BDMC-ANG) as an enhanced anti-inflammatory therapeutic that penetrates the blood–brain barrier and ameliorates alcohol-induced neuroinflammation. StClPr-BDMC-ANG administration maintains the viability of primary glia and inhibits the ethanol-induced upregulation of crucial inflammatory mediators in the prefrontal and medial cortex in a mouse model of chronic ethanol consumption. StClPr-BDMC-ANG treatment also suppresses the ethanol-mediated downregulation of microRNAs known to negatively modulate neuroinflammation in the brain cortex (miRs 146a-5p and let-7b-5p). In summary, our results demonstrate the attenuation of alcohol-induced neuroinflammation by an optimized and targeted polypeptide-based nanoconjugate of a curcuminoid.
Alcohol abuse induces the expression of inflammatory mediators by activating the immune receptors to trigger neuroinflammation and brain damage; however, therapies that reduce neuroimmune system activation may protect against alcohol’s damaging effects. Curcuminoids possess anti-inflammatory properties but suffer from low bioavailability; therefore, we designed a new receptor-targeted biodegradable star-shaped crosslinked polypeptide polymer that bears propargylamine moieties and bisdemethoxycurcumin (StClPr-BDMC-ANG) as an enhanced anti-inflammatory therapeutic that penetrates the blood–brain barrier and ameliorates alcohol-induced neuroinflammation. StClPr-BDMC-ANG administration maintains the viability of primary glia and inhibits the ethanol-induced upregulation of crucial inflammatory mediators in the prefrontal and medial cortex in a mouse model of chronic ethanol consumption. StClPr-BDMC-ANG treatment also suppresses the ethanol-mediated downregulation of microRNAs known to negatively modulate neuroinflammation in the brain cortex (miRs 146a-5p and let-7b-5p). In summary, our results demonstrate the attenuation of alcohol-induced neuroinflammation by an optimized and targeted polypeptide-based nanoconjugate of a curcuminoid.
A. Duro-Castano, A. Sousa-Herves, A. Armiñán, D. Charbonnier. J.J. Arroyo-Crespo, S. Wedepohl, M. Calderón*, M. J. Vicent*. Polyglutamic acid-based crosslinked doxorubicin nanogels as an anti-metastatic treatment for triple negative breast cancer. J. Control. Rel. 2021;332:10-20. [Journal Site][PubMed][Repository Link][Front Page Image]
 Treatment of triple negative breast cancer (TNBC)-associated metastasis represents an unmet clinical need, and we lack effective therapeutics for a disease that exhibits high relapse rates and associates with poor patient outcomes. Advanced nanosized drug delivery systems may enhance the efficacy of first-line chemotherapeutics by altering drug pharmacokinetics and enhancing tumor/metastasis targeting to significantly improve efficacy and safety. Herein, we propose the application of injectable poly-amino acid-based nanogels (NGs) as a versatile hydrophilic drug delivery platform for the treatment of TNBC lung metastasis. We prepared biocompatible and biodegradable cross-linked NGs from polyglutamic acid (PGA) loaded with the chemotherapeutic agent doxorubicin (DOX). Our optimized synthetic procedures generated NGs of ~100 nm in size and 25 wt% drug loading content that became rapidly internalized in TNBC cell lines and displayed IC50 values comparable to the free form of DOX. Importantly, PGA-DOX NGs significantly inhibited lung metastases and almost completely suppressed lymph node metastases in a spontaneously metastatic orthotopic mouse TNBC model. Overall, our newly developed PGA-DOX NGs represent a potentially effective therapeutic strategy for the treatment of TNBC metastases.
Treatment of triple negative breast cancer (TNBC)-associated metastasis represents an unmet clinical need, and we lack effective therapeutics for a disease that exhibits high relapse rates and associates with poor patient outcomes. Advanced nanosized drug delivery systems may enhance the efficacy of first-line chemotherapeutics by altering drug pharmacokinetics and enhancing tumor/metastasis targeting to significantly improve efficacy and safety. Herein, we propose the application of injectable poly-amino acid-based nanogels (NGs) as a versatile hydrophilic drug delivery platform for the treatment of TNBC lung metastasis. We prepared biocompatible and biodegradable cross-linked NGs from polyglutamic acid (PGA) loaded with the chemotherapeutic agent doxorubicin (DOX). Our optimized synthetic procedures generated NGs of ~100 nm in size and 25 wt% drug loading content that became rapidly internalized in TNBC cell lines and displayed IC50 values comparable to the free form of DOX. Importantly, PGA-DOX NGs significantly inhibited lung metastases and almost completely suppressed lymph node metastases in a spontaneously metastatic orthotopic mouse TNBC model. Overall, our newly developed PGA-DOX NGs represent a potentially effective therapeutic strategy for the treatment of TNBC metastases.
D. Van Lysebetten, A. Malfanti, K. Deswarte, K. Koynov, B. Golba, T. Ye, Z. Zhong, S. Kasmi, A. Lamoot, Y. Chen, S. Van Herck, B. N. Lambrecht, N. N. Sanders, S. Lienenklaus, S. A. David, M. J. Vicent, S. De Koker, B. G. De Geest. Lipid-Polyglutamate Nanoparticle Vaccine Platform. ACS Applied Materials & Interfaces. 2021;13:6011-6022. [PubMed][Journal Site][Repository Link]
 Peptide-based subunit vaccines are attractive in view of personalized cancer vaccination with neo-antigens, as well as for the design of the newest generation of vaccines against infectious diseases. Key to mounting robust antigen-specific immunity is delivery of antigen to antigen-presenting (innate immune) cells in lymphoid tissue with concomitant innate immune activation to promote antigen presentation to T cells and to shape the amplitude and nature of the immune response. Nanoparticles that co-deliver both peptide antigen and molecular adjuvants are well suited for this task. However, in the context of peptide-based antigen, an unmet need exists for a generic strategy that allows for co-encapsulation of peptide and molecular adjuvants due to the stark variation in physicochemical properties based on the amino acid sequence of the peptide. These properties also strongly differ from those of many molecular adjuvants. Here, we devise a lipid nanoparticle (LNP) platform that addresses these issues. Key in our concept is poly(l-glutamic acid) (PGA), which serves as a hydrophilic backbone for conjugation of, respectively, peptide antigen (Ag) and an imidazoquinoline (IMDQ) TLR7/8 agonist as a molecular adjuvant. Making use of the PGA’s polyanionic nature, we condensate PGA-Ag and PGA-IMDQ into LNP by electrostatic interaction with an ionizable lipid. We show in vitro and in vivo in mouse models that LNP encapsulation favors uptake by innate immune cells in lymphoid tissue and promotes the induction of Ag-specific T cells responses both after subcutaneous and intravenous administration.
Peptide-based subunit vaccines are attractive in view of personalized cancer vaccination with neo-antigens, as well as for the design of the newest generation of vaccines against infectious diseases. Key to mounting robust antigen-specific immunity is delivery of antigen to antigen-presenting (innate immune) cells in lymphoid tissue with concomitant innate immune activation to promote antigen presentation to T cells and to shape the amplitude and nature of the immune response. Nanoparticles that co-deliver both peptide antigen and molecular adjuvants are well suited for this task. However, in the context of peptide-based antigen, an unmet need exists for a generic strategy that allows for co-encapsulation of peptide and molecular adjuvants due to the stark variation in physicochemical properties based on the amino acid sequence of the peptide. These properties also strongly differ from those of many molecular adjuvants. Here, we devise a lipid nanoparticle (LNP) platform that addresses these issues. Key in our concept is poly(l-glutamic acid) (PGA), which serves as a hydrophilic backbone for conjugation of, respectively, peptide antigen (Ag) and an imidazoquinoline (IMDQ) TLR7/8 agonist as a molecular adjuvant. Making use of the PGA’s polyanionic nature, we condensate PGA-Ag and PGA-IMDQ into LNP by electrostatic interaction with an ionizable lipid. We show in vitro and in vivo in mouse models that LNP encapsulation favors uptake by innate immune cells in lymphoid tissue and promotes the induction of Ag-specific T cells responses both after subcutaneous and intravenous administration.
R. Martí-Centelles, I. Dolz-Pérez, J. De la O, I. Ontoria-Oviedo, P. Sepúlveda, V.J. Nebot*, M. J. Vicent*, and B. Escuder*. Two-Component Peptidic Molecular Gels for Topical Drug Delivery of Naproxen. ACS Applied Bio Materials. 2021;4:935-944. [Journal Site]
 Transdermal drug delivery (TDD) is an advantageous and effective approach for the localized delivery of drugs; however, overcoming the high impermeability of the outermost layer of skin, the stratum corneum, represents a significant challenge to TDD. Herein, we describe a simple and biocompatible platform based on a two-component molecular hydrogel for the transdermal delivery of the non-steroidal anti-inflammatory drug (S)-naproxen. The hydrogel is formed by two amphipathic tetrapeptides bearing aromatic side groups and oppositely-charged residues that co-assemble into fibrillar networks at pH 7.4. We demonstrate that (S)-naproxen, which possesses an aromatic region and an ionizable group, can be effectively loaded into the hydrogel. We characterized drug-loaded hydrogels by NMR and rheology and studied in vitro release under physiologically relevant conditions. Moreover, TDD studies on human skin samples demonstrated a twofold increase in the permeation of (S)-naproxen, which could be advantageous for the localized delivery of the drug.
Transdermal drug delivery (TDD) is an advantageous and effective approach for the localized delivery of drugs; however, overcoming the high impermeability of the outermost layer of skin, the stratum corneum, represents a significant challenge to TDD. Herein, we describe a simple and biocompatible platform based on a two-component molecular hydrogel for the transdermal delivery of the non-steroidal anti-inflammatory drug (S)-naproxen. The hydrogel is formed by two amphipathic tetrapeptides bearing aromatic side groups and oppositely-charged residues that co-assemble into fibrillar networks at pH 7.4. We demonstrate that (S)-naproxen, which possesses an aromatic region and an ionizable group, can be effectively loaded into the hydrogel. We characterized drug-loaded hydrogels by NMR and rheology and studied in vitro release under physiologically relevant conditions. Moreover, TDD studies on human skin samples demonstrated a twofold increase in the permeation of (S)-naproxen, which could be advantageous for the localized delivery of the drug.
A. Lepland, Anni, E. Asciutto, A. Malfanti, L. Simón-Gracia, V. Sidorenko, M.J. Vicent, T. Teesalu, P. Scodeller. Targeting pro-tumoral macrophages in early primary and metastatic breast tumors with CD206-binding mUNO peptide. Molecular Pharmaceutics, 2020(17)7:2518–2531. [Journal Site][PubMed]
 M2-like tumor-associated macrophages (M2 TAMs) play important roles in resistance of tumors to immunotherapies. Selective depletion or reprogramming of M2 TAMs may sensitize the nonresponsive tumors for immune-mediated eradication. However, precision delivery of payloads to M2 TAMs while sparing healthy tissues has remained an unresolved challenge. Here, we studied the application of a short linear peptide (CSPGAK, “mUNO”) for delivery of molecular and nanoscale cargoes in M2 TAMs in vitro and the relevance of the peptide for in vivo targeting of early-stage primary breast tumors and metastatic lung foci. First, we performed in silico modeling and found that mUNO interacts with mouse CD206 via a binding site between lectin domains CTLD1 and CTLD2 – the same site previously demonstrated to be involved in mUNO binding to human CD206. Second, we showed that cultured M2 macrophages take up fluorescein-labeled (FAM) Polymersomes conjugated with mUNO using the sulfhydryl group of its N-terminal cysteine. Pulse-chase studies of FAM-mUNO in M2 macrophages suggested that the peptide avoided lysosomal entrapment and escaped from early endosomes. Third, our in vivo studies with FAM-mUNO demonstrated that intraperitoneal administration results in better pharmacokinetics and higher blood bioavailability than can be achieved with intravenous administration. Intraperitoneal FAM-mUNO, but not FAM-control, showed a robust accumulation in M2-skewed macrophages in mouse models of early primary breast tumor and lung metastasis. This targeting was specific, as no uptake was observed in nonmalignant control organs, including liver, or other cell types in tumor, including M1 macrophages. Collectively, our studies support the application of CD206-binding mUNO peptide for delivery of molecular and nanoscale cargoes to M2 macrophages and manifest the relevance of this mode of targeting primary and metastatic breast tumors.
M2-like tumor-associated macrophages (M2 TAMs) play important roles in resistance of tumors to immunotherapies. Selective depletion or reprogramming of M2 TAMs may sensitize the nonresponsive tumors for immune-mediated eradication. However, precision delivery of payloads to M2 TAMs while sparing healthy tissues has remained an unresolved challenge. Here, we studied the application of a short linear peptide (CSPGAK, “mUNO”) for delivery of molecular and nanoscale cargoes in M2 TAMs in vitro and the relevance of the peptide for in vivo targeting of early-stage primary breast tumors and metastatic lung foci. First, we performed in silico modeling and found that mUNO interacts with mouse CD206 via a binding site between lectin domains CTLD1 and CTLD2 – the same site previously demonstrated to be involved in mUNO binding to human CD206. Second, we showed that cultured M2 macrophages take up fluorescein-labeled (FAM) Polymersomes conjugated with mUNO using the sulfhydryl group of its N-terminal cysteine. Pulse-chase studies of FAM-mUNO in M2 macrophages suggested that the peptide avoided lysosomal entrapment and escaped from early endosomes. Third, our in vivo studies with FAM-mUNO demonstrated that intraperitoneal administration results in better pharmacokinetics and higher blood bioavailability than can be achieved with intravenous administration. Intraperitoneal FAM-mUNO, but not FAM-control, showed a robust accumulation in M2-skewed macrophages in mouse models of early primary breast tumor and lung metastasis. This targeting was specific, as no uptake was observed in nonmalignant control organs, including liver, or other cell types in tumor, including M1 macrophages. Collectively, our studies support the application of CD206-binding mUNO peptide for delivery of molecular and nanoscale cargoes to M2 macrophages and manifest the relevance of this mode of targeting primary and metastatic breast tumors.
G. Córdoba-David, A, Duro-Castano, R.C. Castelo-Branco, C.González-Guerrero, P. Cannata, A.B. Sanz, M.J. Vicent, A. Ortiz & A.M. Ramos. Effective Nephroprotection Against Acute Kidney Injury with a Star-Shaped Polyglutamate-Curcuminoid Conjugate. Scientific Reports, 2020(10):2056. [Journal Site][PubMed]
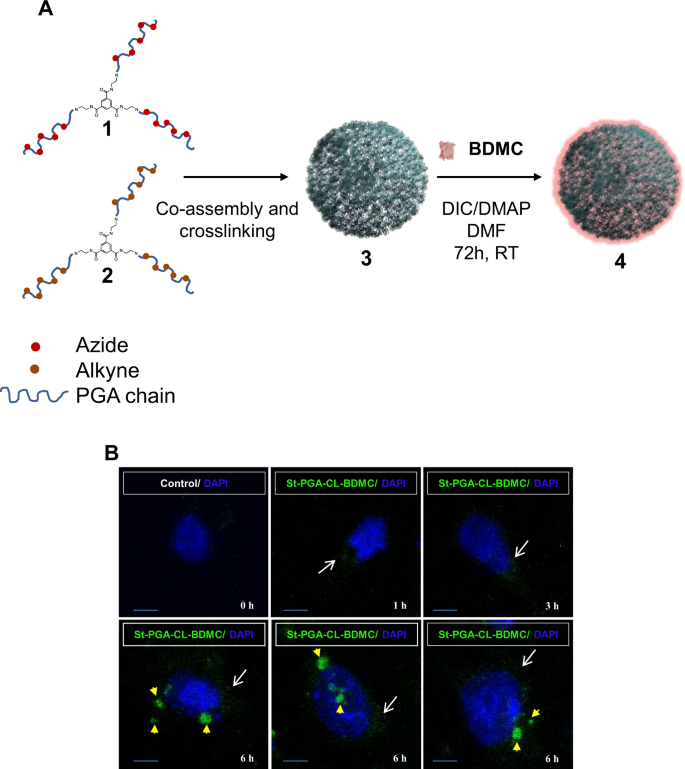 The lack of effective pharmacological treatments for acute kidney injury (AKI) remains a significant public health problem. Given the involvement of apoptosis and regulated necrosis in the initiation and progression of AKI, the inhibition of cell death may contribute to AKI prevention/recovery. Curcuminoids are a family of plant polyphenols that exhibit attractive biological properties that make them potentially suitable for AKI treatment. Now, in cultured tubular cells, we demonstrated that a crosslinked self-assembled star-shaped polyglutamate (PGA) conjugate of bisdemethoxycurcumin (St-PGA-CL-BDMC) inhibits apoptosis and necroptosis induced by Tweak/TNFα/IFNγ alone or concomitant to caspase inhibition. St-PGA-CL-BDMC also reduced NF-κB activation and subsequent gene transcription. In vivo, St-PGA-CL-BDMC prevented renal cell loss and preserved renal function in mice with folic acid-induced AKI. Mechanistically, St-PGA-CL-BDMC inhibited AKI-induced apoptosis and expression of ferroptosis markers and also decreased the kidney expression of genes involved in tubular damage and inflammation, while preserving the kidney expression of the protective factor, Klotho. Thus, due to renal accumulation and attractive pharmacological properties, the application of PGA-based therapeutics may improve nephroprotective properties of current AKI treatments.
The lack of effective pharmacological treatments for acute kidney injury (AKI) remains a significant public health problem. Given the involvement of apoptosis and regulated necrosis in the initiation and progression of AKI, the inhibition of cell death may contribute to AKI prevention/recovery. Curcuminoids are a family of plant polyphenols that exhibit attractive biological properties that make them potentially suitable for AKI treatment. Now, in cultured tubular cells, we demonstrated that a crosslinked self-assembled star-shaped polyglutamate (PGA) conjugate of bisdemethoxycurcumin (St-PGA-CL-BDMC) inhibits apoptosis and necroptosis induced by Tweak/TNFα/IFNγ alone or concomitant to caspase inhibition. St-PGA-CL-BDMC also reduced NF-κB activation and subsequent gene transcription. In vivo, St-PGA-CL-BDMC prevented renal cell loss and preserved renal function in mice with folic acid-induced AKI. Mechanistically, St-PGA-CL-BDMC inhibited AKI-induced apoptosis and expression of ferroptosis markers and also decreased the kidney expression of genes involved in tubular damage and inflammation, while preserving the kidney expression of the protective factor, Klotho. Thus, due to renal accumulation and attractive pharmacological properties, the application of PGA-based therapeutics may improve nephroprotective properties of current AKI treatments.
O. Zagorodko, V. J. Nebot*, and M. J. Vicent*. The generation of stabilized supramolecular nanorods from star-shaped polyglutamates. Polymer Chemistry, 2020(11):1220-1229. [Journal Site][Zenodo]
 We developed a new strategy of polyglutamate nanorod preparation based on supramolecular polymers stabilized with hydrophobic drugs. Using this strategy, we prepared a family of star-shaped polyglutamates (star-PGAs) with benzenetricarboxamide (BTA)-based cores of different hydrophobicity. We then studied the self-assembly of the resulting polymers in aqueous solutions containing a physiological level of salt using fluorescence spectroscopy, small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). We discovered that star-PGAs behave as classical polyelectrolytes in very dilute solutions; however, compounds with hydrophobic cores assembled into one dimensional-nanorods upon an increase in concentration due to supramolecular interactions in the core. Small hydrophobic drugs, such as doxorubicin and irinotecan, stabilized the nanorods and inhibited their disassembly at concentrations below the critical aggregation concentration (CAC). We anticipate that this simple nanorod preparation strategy from star-PGAs will enable the development of new nanomedicines with unique biodistribution profiles and biological activity.
We developed a new strategy of polyglutamate nanorod preparation based on supramolecular polymers stabilized with hydrophobic drugs. Using this strategy, we prepared a family of star-shaped polyglutamates (star-PGAs) with benzenetricarboxamide (BTA)-based cores of different hydrophobicity. We then studied the self-assembly of the resulting polymers in aqueous solutions containing a physiological level of salt using fluorescence spectroscopy, small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). We discovered that star-PGAs behave as classical polyelectrolytes in very dilute solutions; however, compounds with hydrophobic cores assembled into one dimensional-nanorods upon an increase in concentration due to supramolecular interactions in the core. Small hydrophobic drugs, such as doxorubicin and irinotecan, stabilized the nanorods and inhibited their disassembly at concentrations below the critical aggregation concentration (CAC). We anticipate that this simple nanorod preparation strategy from star-PGAs will enable the development of new nanomedicines with unique biodistribution profiles and biological activity.
Dolz-Pérez, I., M. A. Sallam, E. Masiá, D. Morelló-Bolumar, M. D. P. del Caz, P. Graff, D. Abdelmonsif, S. Hedtrich, V. J. Nebot, and M. J. Vicent. Polypeptide-corticosteroid conjugates as a topical treatment approach to psoriasis. Journal of Controlled Release, 2020(318):210-222. [Journal Website][PubMed].
 Topical treatment of mild-to-moderate psoriasis with corticosteroids suffers from challenges that include reduced drug bioavailability at the desired site of action. The retention of therapeutics within the epidermis can safely treat skin inflammation, scaling, and erythema associated with psoriasis while avoiding possible side effects associated with systemic treatments. We successfully synthesized and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-fluocinolone acetonide (FLUO) conjugate that allows the controlled release of the FLUO to reduce skin inflammation. Additionally, the application of a hyaluronic acid (HA)-poly-L-glutamate cross polymer (HA-CP) vehicle boosted skin permeation. During in vitro and ex vivo analyses, we discovered that PGA-FLUO inhibited pro-inflammatory cytokine release, suggesting that polypeptidic conjugation fails to affect the anti-inflammatory activity of FLUO. Additionally, ex vivo human skin permeation studies using confocal microscopy revealed the presence of PGA-FLUO within the epidermis, but a minimal presence in the dermis, thereby reducing the likelihood of FLUO entering the systemic circulation. Finally, we demonstrated that PGA-FLUO applied within HA-CP effectively reduced psoriasis-associated phenotypes in an in vivo mouse model of human psoriasis while also lowering levels of pro-inflammatory cytokines in tissue and serum. Overall, our experimental results demonstrate that PGA-FLUO within an HA-CP penetration enhancer represents an effective topical treatment for psoriasis.
Topical treatment of mild-to-moderate psoriasis with corticosteroids suffers from challenges that include reduced drug bioavailability at the desired site of action. The retention of therapeutics within the epidermis can safely treat skin inflammation, scaling, and erythema associated with psoriasis while avoiding possible side effects associated with systemic treatments. We successfully synthesized and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-fluocinolone acetonide (FLUO) conjugate that allows the controlled release of the FLUO to reduce skin inflammation. Additionally, the application of a hyaluronic acid (HA)-poly-L-glutamate cross polymer (HA-CP) vehicle boosted skin permeation. During in vitro and ex vivo analyses, we discovered that PGA-FLUO inhibited pro-inflammatory cytokine release, suggesting that polypeptidic conjugation fails to affect the anti-inflammatory activity of FLUO. Additionally, ex vivo human skin permeation studies using confocal microscopy revealed the presence of PGA-FLUO within the epidermis, but a minimal presence in the dermis, thereby reducing the likelihood of FLUO entering the systemic circulation. Finally, we demonstrated that PGA-FLUO applied within HA-CP effectively reduced psoriasis-associated phenotypes in an in vivo mouse model of human psoriasis while also lowering levels of pro-inflammatory cytokines in tissue and serum. Overall, our experimental results demonstrate that PGA-FLUO within an HA-CP penetration enhancer represents an effective topical treatment for psoriasis.
Conejos-Sánchez, I., Gallon, E., Niño-Pariente, A., Smith, J. A., De la Fuente, A. G., DiCanio, L., Pluchino, S., Franklin, R.J.M., M.J. Vicent. Polyornithine-based polyplexes to boost effective gene silencing in CNS disorders. Nanoscale, 2020(12):6285-6299. [Nanoscale][PubMed][Front Cover Image]
 Gene silencing therapies have successfully suppressed the translation of target proteins, a strategy that holds great promise for the treatment of central nervous system (CNS) disorders. Advances in the current knowledge on multimolecular delivery vehicles are concentrated on overcoming the difficulties in delivery of small interfering (si)RNA to target tissues, which include anatomical accessibility, slow diffusion, safety concerns, and the requirement for specific cell uptake within the unique environment of the CNS. The present work addressed these challenges through the implementation of polyornithine derivatives in the construction of polyplexes used as non-viral siRNA delivery vectors. Physicochemical and biological characterization revealed biodegradability and biocompatibility of our polyornithine-based system and the ability to silence gene expression in primary oligodendrocyte progenitor cells (OPCs) effectively. In summary, the well-defined properties and neurological compatibility of this polypeptide-based platform highlight its potential utility in the treatment of CNS disorders.
Gene silencing therapies have successfully suppressed the translation of target proteins, a strategy that holds great promise for the treatment of central nervous system (CNS) disorders. Advances in the current knowledge on multimolecular delivery vehicles are concentrated on overcoming the difficulties in delivery of small interfering (si)RNA to target tissues, which include anatomical accessibility, slow diffusion, safety concerns, and the requirement for specific cell uptake within the unique environment of the CNS. The present work addressed these challenges through the implementation of polyornithine derivatives in the construction of polyplexes used as non-viral siRNA delivery vectors. Physicochemical and biological characterization revealed biodegradability and biocompatibility of our polyornithine-based system and the ability to silence gene expression in primary oligodendrocyte progenitor cells (OPCs) effectively. In summary, the well-defined properties and neurological compatibility of this polypeptide-based platform highlight its potential utility in the treatment of CNS disorders.
Arroyo‐Crespo, J. J., Armiñán, A.* , Charbonnier, D., Deladriere, C., Palomino‐Schätzlein, M. , Lamas‐Domingo, R. , Forteza, J. , Pineda‐Lucena, A. and Vicent, M. J.* Characterization of Triple‐Negative Breast Cancer Preclinical Models Provides Functional Evidence of Metastatic Progression. International Journal of Cancer, 2019;145(8):2267-2281. [PubMed][Journal Site][Zenodo]
 Triple‐negative breast cancer (TNBC), an aggressive, metastatic, and recurrent breast cancer (BC) subtype, currently suffers from a lack of adequately described spontaneously metastatic preclinical models that faithfully reproduce the clinical scenario. We describe two preclinical spontaneously metastatic TNBC orthotopic murine models for the development of advanced therapeutics: an immunodeficient human MDA‐MB‐231‐Luc model and an immunocompetent mouse 4T1 model. Furthermore, we provide a broad range of multifactorial analysis for both models that could provide relevant information for the development of new therapies and diagnostic tools. Our comparisons uncovered differential growth rates, stromal arrangements, and metabolic profiles in primary tumors, and the presence of cancer‐associated adipocyte infiltration in the MDA‐MB‐231‐Luc model. Histopathological studies highlighted the more rapid metastatic spread to the lungs in the 4T1 model following a lymphatic route, while we observed both homogeneous (MDA‐MB‐231‐Luc) and heterogeneous (4T1) metastatic spread to axillary lymph nodes. We encountered unique metabolomic signatures in each model, including crucial amino acids and cell membrane components. Hematological analysis demonstrated severe leukemoid and lymphoid reactions in the 4T1 model with the partial reestablishment of immune responses in the immunocompromised MDA‐MB‐231‐Luc model. Additionally, we discovered β‐immunoglobulinemia and increased basal levels of G‐CSF correlating with a metastatic switch, with G‐CSF also promoting extramedullary hematopoiesis (both models) and causing hepatosplenomegaly (4T1 model). Overall, we believe that the characterization of these preclinical models will foster the development of advanced therapeutic strategies for TNBC treatment, especially for the treatment of patients presenting both, primary tumors and metastatic spread.
Triple‐negative breast cancer (TNBC), an aggressive, metastatic, and recurrent breast cancer (BC) subtype, currently suffers from a lack of adequately described spontaneously metastatic preclinical models that faithfully reproduce the clinical scenario. We describe two preclinical spontaneously metastatic TNBC orthotopic murine models for the development of advanced therapeutics: an immunodeficient human MDA‐MB‐231‐Luc model and an immunocompetent mouse 4T1 model. Furthermore, we provide a broad range of multifactorial analysis for both models that could provide relevant information for the development of new therapies and diagnostic tools. Our comparisons uncovered differential growth rates, stromal arrangements, and metabolic profiles in primary tumors, and the presence of cancer‐associated adipocyte infiltration in the MDA‐MB‐231‐Luc model. Histopathological studies highlighted the more rapid metastatic spread to the lungs in the 4T1 model following a lymphatic route, while we observed both homogeneous (MDA‐MB‐231‐Luc) and heterogeneous (4T1) metastatic spread to axillary lymph nodes. We encountered unique metabolomic signatures in each model, including crucial amino acids and cell membrane components. Hematological analysis demonstrated severe leukemoid and lymphoid reactions in the 4T1 model with the partial reestablishment of immune responses in the immunocompromised MDA‐MB‐231‐Luc model. Additionally, we discovered β‐immunoglobulinemia and increased basal levels of G‐CSF correlating with a metastatic switch, with G‐CSF also promoting extramedullary hematopoiesis (both models) and causing hepatosplenomegaly (4T1 model). Overall, we believe that the characterization of these preclinical models will foster the development of advanced therapeutic strategies for TNBC treatment, especially for the treatment of patients presenting both, primary tumors and metastatic spread.
Arroyo-Crespo, J.J., Armiñán, A., Charbonnier, D., Balzano-Nogueira, L., Huertas-López, F., Martí, C., Tarazona, S., Forteza, J., Conesa, A., Vicent, M.J*. Tumor microenvironment-targeted poly-L-glutamic acid-based combination conjugate for enhanced triple negative breast cancer treatment. Biomaterials, 2018. 186: p8-21. [PubMed][Free Download at Biomaterials].
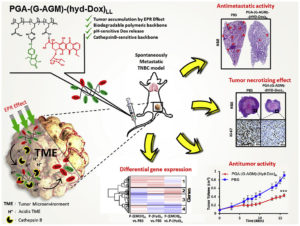 The intrinsic characteristics of the tumor microenvironment (TME), including acidic pH and overexpression of hydrolytic enzymes, offer an exciting opportunity for the rational design of TME-drug delivery systems (DDS). We developed and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-based combination conjugate family with the aim of optimizing anticancer effects. We obtained combination conjugates bearing Doxorubicin (Dox) and aminoglutethimide (AGM) with two Dox loadings and two different hydrazone pH-sensitive linkers that promote the specific release of Dox from the polymeric backbone within the TME. Low Dox loading coupled with a short hydrazone linker yielded optimal effects on primary tumor growth, lung metastasis (∼90% reduction), and toxicological profile in a preclinical metastatic triple-negative breast cancer (TNBC) murine model. The use of transcriptomic analysis helped us to identify the molecular mechanisms responsible for such results including a differential immunomodulation and cell death pathways among the conjugates. This data highlights the advantages of targeting the TME, the therapeutic value of polymer-based combination approaches, and the utility of –omics-based analysis to accelerate anticancer DDS.
The intrinsic characteristics of the tumor microenvironment (TME), including acidic pH and overexpression of hydrolytic enzymes, offer an exciting opportunity for the rational design of TME-drug delivery systems (DDS). We developed and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-based combination conjugate family with the aim of optimizing anticancer effects. We obtained combination conjugates bearing Doxorubicin (Dox) and aminoglutethimide (AGM) with two Dox loadings and two different hydrazone pH-sensitive linkers that promote the specific release of Dox from the polymeric backbone within the TME. Low Dox loading coupled with a short hydrazone linker yielded optimal effects on primary tumor growth, lung metastasis (∼90% reduction), and toxicological profile in a preclinical metastatic triple-negative breast cancer (TNBC) murine model. The use of transcriptomic analysis helped us to identify the molecular mechanisms responsible for such results including a differential immunomodulation and cell death pathways among the conjugates. This data highlights the advantages of targeting the TME, the therapeutic value of polymer-based combination approaches, and the utility of –omics-based analysis to accelerate anticancer DDS.
Plyduang, T., Armiñán, A., Movellan, J., England, R.M., Wiwattanapatapee, R., and Vicent, M.J.*, Polyacetal-Based Combination Therapy for the Treatment of Prostate Cancer. Macromolecular Rapid Communications, 2018. 39: p1800265. [PubMed].
 The high incidence of prostate carcinogenesis has prompted the search for novel effective treatment approaches. We have employed curcumin (Curc) and diethylstilbestrol (DES) to synthesize a series of polyacetal (PA)‐based combination conjugates for prostate cancer (PCa) treatment. Given their bihydroxyl functionalities, Curc and DES molecules were incorporated into a PA mainchain using a one‐pot reaction between diols and divinyl ethers. The PA‐conjugates released both drugs under acidic conditions, such as those found in the tumor microenvironment, endosomes, or lysosomes, while remaining stable at neutral pH 7.4. The drug ratio was optimized to achieve anticancer drug synergism with elevated cytotoxicity against LNCaP‐hormone‐dependent human PCa cells conferred via the induction of S phase cell cycle arrest by the upregulation of p53 and CDK inhibitors p21Waf/CIP1 and downregulation of cyclin D1. The application of rationally designed PA‐Curc‐DES combination conjugates represents a potentially exciting new treatment for prostate cancer.
The high incidence of prostate carcinogenesis has prompted the search for novel effective treatment approaches. We have employed curcumin (Curc) and diethylstilbestrol (DES) to synthesize a series of polyacetal (PA)‐based combination conjugates for prostate cancer (PCa) treatment. Given their bihydroxyl functionalities, Curc and DES molecules were incorporated into a PA mainchain using a one‐pot reaction between diols and divinyl ethers. The PA‐conjugates released both drugs under acidic conditions, such as those found in the tumor microenvironment, endosomes, or lysosomes, while remaining stable at neutral pH 7.4. The drug ratio was optimized to achieve anticancer drug synergism with elevated cytotoxicity against LNCaP‐hormone‐dependent human PCa cells conferred via the induction of S phase cell cycle arrest by the upregulation of p53 and CDK inhibitors p21Waf/CIP1 and downregulation of cyclin D1. The application of rationally designed PA‐Curc‐DES combination conjugates represents a potentially exciting new treatment for prostate cancer.
Duro-Castano, A., Lim, N.H., Tranchant, I., Amoura, M., Beau, F., Wieland, H., Kingler, O., Herrmann, M., Nazaré, M., Plettenburg, O., Dive, V.*, Vicent, M.J.*, and Nagase, H*. In Vivo Imaging of MMP-13 Activity Using a Specific Polymer-FRET Peptide Conjugate Detects Early Osteoarthritis and Inhibitor Efficacy. Advanced Functional Materials, 2018. 28: p1802738. [Advanced Functional Materials].
 Imaging early molecular changes in osteoarthritic (OA) joints is instrumental for the development of disease‐modifying drugs. To this end, a fluorescent resonance energy transfer‐based peptide probe that is cleavable by matrix metalloproteinase 13 (MMP‐13) has been developed. This protease degrades type II collagen, a major matrix component of cartilage. The probe exhibits high catalytic efficiency (kcat/KM = 6.5 × 105m−1 s−1) and high selectivity for MMP‐13 over a set of nine MMPs. To achieve optimal in vivo pharmacokinetics and tissue penetration, the probe has been further conjugated to a linear l‐polyglutamate chain of 30 kDa. The conjugate detects early biochemical events that occur in a surgically induced murine model of OA before major histological changes. The nanometric probe is suitable for the monitoring of in vivo efficacy of an orally bioavailable MMP‐13 inhibitor, which effectively blocks cartilage degradation during the development of OA. This new polymer‐probe can therefore be a useful tool in detecting early OA, disease progression, and in developing MMP‐13‐based disease‐modifying drugs for OA.
Imaging early molecular changes in osteoarthritic (OA) joints is instrumental for the development of disease‐modifying drugs. To this end, a fluorescent resonance energy transfer‐based peptide probe that is cleavable by matrix metalloproteinase 13 (MMP‐13) has been developed. This protease degrades type II collagen, a major matrix component of cartilage. The probe exhibits high catalytic efficiency (kcat/KM = 6.5 × 105m−1 s−1) and high selectivity for MMP‐13 over a set of nine MMPs. To achieve optimal in vivo pharmacokinetics and tissue penetration, the probe has been further conjugated to a linear l‐polyglutamate chain of 30 kDa. The conjugate detects early biochemical events that occur in a surgically induced murine model of OA before major histological changes. The nanometric probe is suitable for the monitoring of in vivo efficacy of an orally bioavailable MMP‐13 inhibitor, which effectively blocks cartilage degradation during the development of OA. This new polymer‐probe can therefore be a useful tool in detecting early OA, disease progression, and in developing MMP‐13‐based disease‐modifying drugs for OA.
Cheah HY, Gallon E, Dumoulin F, Hoe SZ, Japundžić-Žigon N, Glumac S, Lee HB, Anand P, Chung LY, Vicent MJ*, Kiew LV*. Near-infrared activatable phthalocyanine-poly-L-glutamic acid conjugate: enhanced in vivo safety and antitumor efficacy towards an effective photodynamic cancer therapy. Molecular Pharmaceutics, 2018. 15(7): p. 2594–2605. [PubMed].
 We previously developed a new zinc (II) phthalocyanine (ZnPc) derivative (Pc 1) conjugated to poly-L-glutamic acid (PGA) (1-PG) to address the limitations of ZnPc as part of an antitumor photodynamic therapy approach, which include hydrophobicity, phototoxicity and non-selectivity in biodistribution and tumor targeting. During this study, we discovered that 1-PG possessed high near infrared (NIR) light absorptivity (λmax = 675 nm), good singlet oxygen generation efficiency in an aqueous environment, and enhanced photocytotoxic efficacy and cancer cell uptake in vitro. In the current study, we discovered that 1-PG selectively accumulated in 4T1 mouse mammary tumors, with a retention time of up to 48 hours. Furthermore, as part of an antitumor PDT, low dose 1-PG (2 mg Pc 1 equivalent/kg) induced a greater tumor volume reduction (-74 ± 5%) when compared to high dose ZnPc (8 mg/kg, -50 ± 12%). At higher treatment doses (8 mg Pc 1 equivalent/kg), 1-PG reduced tumor volume maximally (-91 ± 6%) and suppressed tumor size to a minimal level for up to 15 days. The kidney, liver, and lungs of the mice treated with 1-PG (both low and high doses) were free from 4T1 tumor metastasis at the end of the study. Telemetry-spectral-echocardiography studies also revealed that PGA (65 mg/kg) produced insignificant changes to the cardiovascular physiology of Wistar-Kyoto rats when administered in vivo. Results indicate that PGA displays an excellent cardiovascular safety profile, underlining its suitability for application as a nano-drug-carrier in vivo. These current findings indicate the potential of 1-PG as a useful photosensitizer candidate for clinical PDT.
We previously developed a new zinc (II) phthalocyanine (ZnPc) derivative (Pc 1) conjugated to poly-L-glutamic acid (PGA) (1-PG) to address the limitations of ZnPc as part of an antitumor photodynamic therapy approach, which include hydrophobicity, phototoxicity and non-selectivity in biodistribution and tumor targeting. During this study, we discovered that 1-PG possessed high near infrared (NIR) light absorptivity (λmax = 675 nm), good singlet oxygen generation efficiency in an aqueous environment, and enhanced photocytotoxic efficacy and cancer cell uptake in vitro. In the current study, we discovered that 1-PG selectively accumulated in 4T1 mouse mammary tumors, with a retention time of up to 48 hours. Furthermore, as part of an antitumor PDT, low dose 1-PG (2 mg Pc 1 equivalent/kg) induced a greater tumor volume reduction (-74 ± 5%) when compared to high dose ZnPc (8 mg/kg, -50 ± 12%). At higher treatment doses (8 mg Pc 1 equivalent/kg), 1-PG reduced tumor volume maximally (-91 ± 6%) and suppressed tumor size to a minimal level for up to 15 days. The kidney, liver, and lungs of the mice treated with 1-PG (both low and high doses) were free from 4T1 tumor metastasis at the end of the study. Telemetry-spectral-echocardiography studies also revealed that PGA (65 mg/kg) produced insignificant changes to the cardiovascular physiology of Wistar-Kyoto rats when administered in vivo. Results indicate that PGA displays an excellent cardiovascular safety profile, underlining its suitability for application as a nano-drug-carrier in vivo. These current findings indicate the potential of 1-PG as a useful photosensitizer candidate for clinical PDT.
Arroyo‐Crespo, J.J., Deladriere, C., Nebot, V.J., Charbonnier, D., Masiá, E., Paul, A., James, C., Armiñán, A.*, and Vicent, M.J.*, Anticancer Activity Driven by Drug Linker Modification in a Polyglutamic Acid‐Based Combination‐Drug Conjugate. Advanced Functional Materials, 2018. 28(22): p. 1800931 [See Full text free at Advanced Functional Materials]
 Combination nanotherapies for the treatment of breast cancer permits synergistic drug targeting of multiple pathways. However, poor carrier degradability, poor synergism of the combined drugs, low drug release regulation, and a lack of control on final macromolecule solution conformation (which drives the biological fate) limit the application of this strategy. The present study describes the development of a family of drug delivery systems composed of chemotherapeutic (doxorubicin) and endocrine therapy (aromatase inhibitor aminoglutethimide) agents conjugated to a biodegradable poly‐l‐glutamic acid backbone via various linking moieties. Data from in vitro cytotoxicity and drug release assessments and animal model validation select a conjugate family member with optimal biological performance. Exhaustive physicochemical characterization in relevant media (including the study of secondary structure, size measurements, and detailed small‐angle neutron scattering analysis) correlates biological data with the intrinsic supramolecular characteristics of the conjugate. Overall, this study demonstrates how a small flexible Gly linker can modify the spatial conformation of the entire polymer–drug conjugate, promote the synergistic release of both drugs, and significantly improve biological activity. These findings highlight the need for a deeper understanding of polymer–drug conjugates at supramolecular level to allow the design of more effective polymer–drug conjugates.
Combination nanotherapies for the treatment of breast cancer permits synergistic drug targeting of multiple pathways. However, poor carrier degradability, poor synergism of the combined drugs, low drug release regulation, and a lack of control on final macromolecule solution conformation (which drives the biological fate) limit the application of this strategy. The present study describes the development of a family of drug delivery systems composed of chemotherapeutic (doxorubicin) and endocrine therapy (aromatase inhibitor aminoglutethimide) agents conjugated to a biodegradable poly‐l‐glutamic acid backbone via various linking moieties. Data from in vitro cytotoxicity and drug release assessments and animal model validation select a conjugate family member with optimal biological performance. Exhaustive physicochemical characterization in relevant media (including the study of secondary structure, size measurements, and detailed small‐angle neutron scattering analysis) correlates biological data with the intrinsic supramolecular characteristics of the conjugate. Overall, this study demonstrates how a small flexible Gly linker can modify the spatial conformation of the entire polymer–drug conjugate, promote the synergistic release of both drugs, and significantly improve biological activity. These findings highlight the need for a deeper understanding of polymer–drug conjugates at supramolecular level to allow the design of more effective polymer–drug conjugates.
Armiñán, A., Palomino-Schätzlein, M., Deladriere, C., Arroyo-Crespo, J. J., Vicente-Ruiz, S., Vicent, M. J.*, and Pineda-Lucena, A.* Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials 2018.162: p. 144-153 [PubMed]
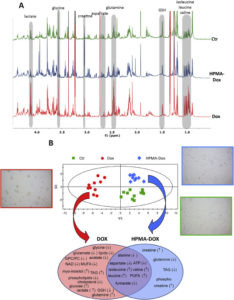 Metabolomics is becoming a relevant tool for understanding the molecular mechanisms involved in the response to new drug delivery systems. The applicability of this experimental approach to cell cultures and animal models makes metabolomics a useful tool for establishing direct connections between in vitro and in vivo data, thus providing a reliable platform for the characterization of chemotherapeutic agents. Herein, we used metabolomic profiles based on nuclear magnetic resonance (NMR) spectroscopy to evaluate the biochemical pathways involved in the response to a chemotherapeutic anthracycline drug (Doxorubicin, Dox) and an N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-conjugated form (HPMA-Dox) in an in vitro cell culture model and an in vivo orthotopic breast cancer model. We also used protein expression and flow cytometry studies to obtain a better coverage of the biochemical alterations associated with the administration of these compounds. The overall analysis revealed that polymer conjugation leads to increased apoptosis, reduced glycolysis, and reduced levels of phospholipids when compared to the free chemotherapeutic drug. Our results represent a first step in the application of integrated in vitro and in vivo metabolomic studies to the evaluation of drug delivery systems.
Metabolomics is becoming a relevant tool for understanding the molecular mechanisms involved in the response to new drug delivery systems. The applicability of this experimental approach to cell cultures and animal models makes metabolomics a useful tool for establishing direct connections between in vitro and in vivo data, thus providing a reliable platform for the characterization of chemotherapeutic agents. Herein, we used metabolomic profiles based on nuclear magnetic resonance (NMR) spectroscopy to evaluate the biochemical pathways involved in the response to a chemotherapeutic anthracycline drug (Doxorubicin, Dox) and an N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-conjugated form (HPMA-Dox) in an in vitro cell culture model and an in vivo orthotopic breast cancer model. We also used protein expression and flow cytometry studies to obtain a better coverage of the biochemical alterations associated with the administration of these compounds. The overall analysis revealed that polymer conjugation leads to increased apoptosis, reduced glycolysis, and reduced levels of phospholipids when compared to the free chemotherapeutic drug. Our results represent a first step in the application of integrated in vitro and in vivo metabolomic studies to the evaluation of drug delivery systems.
Escalona, G.R., Sanchis, J., and Vicent, M.J.* pH-Responsive Polyacetal–Protein Conjugates Designed for Polymer Masked–Unmasked Protein Therapy (PUMPT). Macromolecular Bioscience, 2018. 18(1): p. 1700302 [PubMed]
 Polymer masked–unmasked protein therapy (PUMPT) employs polymer conjugation to protect therapeutic proteins during transit through the bloodstream and allow controlled release at a disease site via triggered degradation of the polymeric component. Most reported PUMPT systems are based on the specific enzymatic degradation of the polymeric component to release the protein and reinstate its activity. In these cases, therapeutic output is dependent on the presence of the required enzyme at the disease site at a sufficiently high concentration. The present study aims to overcome this design limitation by using pH as the protein release trigger. An acidic-pH triggered PUMPT system is described herein employing biodegradable polyacetals (PAs) and trypsin as a model protein. While this system protects trypsin activity at the neutral pH of the bloodstream, acidic pH (characteristic of disease sites, tissue damage, or lysosomal compartments) contributes to PA degradation and the “unmasking” of protein activity.
Polymer masked–unmasked protein therapy (PUMPT) employs polymer conjugation to protect therapeutic proteins during transit through the bloodstream and allow controlled release at a disease site via triggered degradation of the polymeric component. Most reported PUMPT systems are based on the specific enzymatic degradation of the polymeric component to release the protein and reinstate its activity. In these cases, therapeutic output is dependent on the presence of the required enzyme at the disease site at a sufficiently high concentration. The present study aims to overcome this design limitation by using pH as the protein release trigger. An acidic-pH triggered PUMPT system is described herein employing biodegradable polyacetals (PAs) and trypsin as a model protein. While this system protects trypsin activity at the neutral pH of the bloodstream, acidic pH (characteristic of disease sites, tissue damage, or lysosomal compartments) contributes to PA degradation and the “unmasking” of protein activity.
Duro-Castaño, A., Nebot, V. J., Niño-Pariente, A., Armiñán, A., Arroyo-Crespo, J. J., Paul, A., Feiner-Gracia, N., Albertazzi, L. and Vicent, M. J.* Capturing “Extraordinary” Soft-Assembled Charge-Like Polypeptides as a Strategy for Nanocarrier Design. Adv Mater, 2017. 29(39): p. 1702888 [PubMed]
 The rational design of nanomedicines is a challenging task given the complex architectures required for the construction of nanosized carriers with embedded therapeutic properties and the complex interface of these materials with the biological environment. Herein, an unexpected charge-like attraction mechanism of self-assembly for star-shaped polyglutamates in nonsalty aqueous solutions is identified, which matches the ubiquitous “ordinary-extraordinary” phenomenon previously described by physicists. For the first time, a bottom-up methodology for the stabilization of these nanosized soft-assembled star-shaped polyglutamates is also described, enabling the translation of theoretical research into nanomaterials with applicability within the drug-delivery field. Covalent capture of these labile assemblies provides access to unprecedented architectures to be used as nanocarriers. The enhanced in vitro and in vivo properties of these novel nanoconstructs as drug-delivery systems highlight the potential of this approach for tumor-localized as well as lymphotropic delivery.
The rational design of nanomedicines is a challenging task given the complex architectures required for the construction of nanosized carriers with embedded therapeutic properties and the complex interface of these materials with the biological environment. Herein, an unexpected charge-like attraction mechanism of self-assembly for star-shaped polyglutamates in nonsalty aqueous solutions is identified, which matches the ubiquitous “ordinary-extraordinary” phenomenon previously described by physicists. For the first time, a bottom-up methodology for the stabilization of these nanosized soft-assembled star-shaped polyglutamates is also described, enabling the translation of theoretical research into nanomaterials with applicability within the drug-delivery field. Covalent capture of these labile assemblies provides access to unprecedented architectures to be used as nanocarriers. The enhanced in vitro and in vivo properties of these novel nanoconstructs as drug-delivery systems highlight the potential of this approach for tumor-localized as well as lymphotropic delivery.
Armiñán, A., Mendes, L., Carrola, J., Movellan, J., Vicent, M. J. and Duarte, I. F. HIF-1alpha inhibition by diethylstilbestrol and its polyacetal conjugate in hypoxic prostate tumour cells: Insights from NMR metabolomics. J Drug Target, 2017. 25(9-10): p. 845-855. [PubMed]
In this study, we have employed 1H NMR metabolomics to assess the metabolic responses of PC3 prostate tumour cells to hypoxia and to pharmacological HIF-1alpha inhibition by DES or its polyacetal conjugate tert-DES. Oxygen deprivation prompted a number of changes in intracellular composition and metabolic activity, mainly reflecting upregulated glycolysis, amino acid catabolism and other compensatory mechanisms used by hypoxic cells to deal with oxidative imbalance and energy deficit. Cell treatment with a non-cytotoxic concentration of DES, under hypoxia, triggered significant changes in 17 metabolites. Among these, lactate, phosphocreatine and reduced glutathione, whose levels showed opposite variations in hypoxic and drug-treated cells, emerged as possible markers of DES-induced HIF-1alpha inhibition. Furthermore, the free drug had a much higher impact on the cellular metabolome than tert-DES, particularly concerning polyamine and pyrimidine biosynthetic pathways, known to be tightly involved in cell proliferation and growth. This is likely due to the different cell pharmacokinetics observed between free and conjugated DES. Overall, this study has revealed a number of unanticipated metabolic changes that inform on DES and tert-DES direct cellular effects, providing further insight into their mode of action at the biochemical level.
Eldar-Boock, A., Blau, R., Ryppa, C., Baabur-Cohen, H., Many, A., Vicent, M. J., Kratz, F., Sanchis, J. and Satchi-Fainaro, R. Integrin-Targeted Nano-Sized Polymeric Systems for Paclitaxel Conjugation: A Comparative Study. J Drug Target, 2017. 25(9-10): p. 829-844. [PubMed]
The generation of rationally-designed polymer therapeutics via the conjugation of low molecular weight anti-cancer drugs to water-soluble polymeric nanocarriers aims to improve the therapeutic index. Here, we focus on applying polymer therapeutics to target two cell compartments simultaneously – tumor cells and angiogenic endothelial cells. Comparing different polymeric backbones carrying the same therapeutic agent and targeting moiety may shed light on any correlation between the choice of polymer and the anti-cancer activity of the conjugate. Here, we compared three paclitaxel (PTX) bound conjugates with poly-L-glutamic acid (PGA, 4.9 mol%), 2-hydroxypropylmethacrylamide (HPMA, 1.2 mol%) copolymer, or polyethyleneglycol (PEG, 1:1 conjugate). PGA and HPMA copolymer are multivalent polymers that allow the conjugation of multiple compounds within the same polymer backbone, while PEG is a commercially-available Food and Drug Administration (FDA)-approved polymer. We further conjugated PGA-PTX and PEG-PTX with the integrin alphavbeta3-targeting moiety RGD (5.5 mol% and 1:1 conjugate respectively). We based our selection on the overexpression of integrin alphavbeta3 on angiogenic endothelial cells and several types of cancer cells. Our findings suggest that polymer structure has significant effect on the conjugate’s activity on different tumor compartments. A multivalent PGA-PTX-E-[c(RGDfK)2] conjugate displayed a stronger inhibitory effect on the endothelial compartment, with a 50% inhibition of cell migration in HUVEC cells, while a PTX-PEG-E-[c(RGDfK)2] conjugate possessed enhanced anti-cancer activity in MDA-MB-231 tumor cells (IC50 = 20 nM vs IC50 300 nM for the PGA conjugate).
Niño-Pariente, A., Armiñan, A., Reinhard, S., Scholz, C., Wagner, E. and Vicent, M. J.* Design of Poly-l-Glutamate-Based Complexes for pDNA Delivery. Macromolecular Bioscience, 2017. 17(10): p. 1700029-n/a. [PubMed]
 Due to the polyanionic nature of DNA, typically cationic or neutral delivery vehicles have been used for gene delivery. As a new approach, this study focuses on the design, development, and validation of nonviral polypeptide-based carriers for oligonucleotide delivery based on a negatively charged poly-l-glutamic acid (PGA) backbone partly derivatized with oligoaminoamide residues. To this end, PGA-derivatives modified with different pentameric succinyl tetraethylene pentamines (Stp5 ) are designed. Optionally, histidines for modulation of endosomal buffer capacity and cysteines for pDNA complex stabilization are included, followed by characterization of biophysical properties and gene transfer efficiency in N2a neuroblastoma or 4T1 breast cancer cells.
Due to the polyanionic nature of DNA, typically cationic or neutral delivery vehicles have been used for gene delivery. As a new approach, this study focuses on the design, development, and validation of nonviral polypeptide-based carriers for oligonucleotide delivery based on a negatively charged poly-l-glutamic acid (PGA) backbone partly derivatized with oligoaminoamide residues. To this end, PGA-derivatives modified with different pentameric succinyl tetraethylene pentamines (Stp5 ) are designed. Optionally, histidines for modulation of endosomal buffer capacity and cysteines for pDNA complex stabilization are included, followed by characterization of biophysical properties and gene transfer efficiency in N2a neuroblastoma or 4T1 breast cancer cells.
Kiew, L.V., Cheah, H.Y., Voon, S.H., Gallon, E., Movellan, J., Ng, K.H., Alpugan, S., Lee, H.B., Dumoulin, F., Vicent, M.J.*, and Chueldang, L.Y. Near-Infrared Activatable Phthalocyanine-Poly-L-Glutamic Acid Conjugate: Increased Cellular Uptake and Light-Dark Toxicity Ratio Towards An Effective Photodynamic Cancer Therapy. Nanomedicine, 2017. 13(4): p. 1447-1458. [PubMed]
 In photodynamic therapy (PDT), the low absorptivity of photosensitizers in an aqueous environment reduces singlet oxygen generation efficiency and thereby decreases photosensitizing efficacy in biological conditions. To circumvent this problem, we designed a phthalocyanine-poly-L-glutamic acid conjugate (1-PG) made from a new phthalocyanine (Pc 1) monofunctionalized to allow adequate conjugation to PGA. The resulting 1-PG conjugate retained high absorptivity in the near-infrared (NIR) region at its λmax 675 nm in an aqueous environment. The 1-PG conjugate demonstrated good singlet oxygen generation efficiency, increased uptake by 4T1 breast cancer cells via clathrin-mediated endocytosis, and enhanced photocytotoxic efficacy. The conjugate also displayed a high light-dark toxicity ratio, approximately 1.5-fold greater than zinc phthalocyanine at higher concentration (10mM), an important feature for the reduction of dark toxicity and unwanted side effects. These results suggest that the 1-PG conjugate could be a useful alternative for deep tissue treatment with enhanced anti-cancer (PDT) efficacy.
In photodynamic therapy (PDT), the low absorptivity of photosensitizers in an aqueous environment reduces singlet oxygen generation efficiency and thereby decreases photosensitizing efficacy in biological conditions. To circumvent this problem, we designed a phthalocyanine-poly-L-glutamic acid conjugate (1-PG) made from a new phthalocyanine (Pc 1) monofunctionalized to allow adequate conjugation to PGA. The resulting 1-PG conjugate retained high absorptivity in the near-infrared (NIR) region at its λmax 675 nm in an aqueous environment. The 1-PG conjugate demonstrated good singlet oxygen generation efficiency, increased uptake by 4T1 breast cancer cells via clathrin-mediated endocytosis, and enhanced photocytotoxic efficacy. The conjugate also displayed a high light-dark toxicity ratio, approximately 1.5-fold greater than zinc phthalocyanine at higher concentration (10mM), an important feature for the reduction of dark toxicity and unwanted side effects. These results suggest that the 1-PG conjugate could be a useful alternative for deep tissue treatment with enhanced anti-cancer (PDT) efficacy.
Cheah, H.Y., Sarenac, O., Arroyo, J.J., Vasic, M., Lozic, M., Glumac, S., Hoe, S.Z., Hindmarch, C.C., Murphy, D., Kiew, L.V., Lee, H.B., Vicent, M.J., Chung, L.Y., and Japundzic-Zigon, N., Hemodynamic effects of HPMA copolymer based doxorubicin conjugate: A randomized controlled and comparative spectral study in conscious rats. Nanotoxicology, 2017. 11(2): p. 210-222. [PubMed]
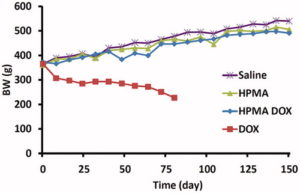 Conjugation of Doxorubicin (DOX) to N-(2-Hydroxypropyl) methylacrylamide copolymer (HPMA) has significantly reduced the DOX associated cardiotoxicity. However, the reports on the impact of HPMADOX conjugates on the cardiovascular system such as blood pressure (BP) and heart rate (HR) were in restrained animals using tail cuff and/or other methods that lacked the resolution and sensitivity. Herein, we employed radiotelemetric-spectral-echocardiography approach to further understand the in vivo cardiovascular hemodynamics and variability post administration of free DOX and HPMA-DOX. Rats implanted with radiotelemetry device were administered intravenously with DOX (5 mg/kg), HPMA-DOX (5 mg DOX equivalent/kg) and HPMA copolymer and subjected to continuous cardiovascular monitoring and echocardiography for 140 days. We found that DOX-treated rats had ruffled fur, reduced body weight and a low survival rate. Although BP and HR were normal, spectral analysis indicated that their BP and HR variabilities were reduced. All rats exhibited typical signs of cardiotoxicity at histopathology. In contrast, HPMA-DOX rats gained weight over time and survived. Although BP, HR and related variabilities were unaffected, the left ventricular end diastolic volume of these rats, as well as of the HPMA copolymer-treated rats, was found increased at the end of observation period. Additionally, HPMA copolymer caused microscopic injury of the heart tissue. All of these suggest the necessity of caution when employing HPMA as carrier for prolonged drug delivery. The current study also indicates the potential of radiotelemetric-spectral-echocardiography approach for improved preclinical cardiovascular risk assessment of polymer-drug conjugate and other nano-sized-drug constructs.
Conjugation of Doxorubicin (DOX) to N-(2-Hydroxypropyl) methylacrylamide copolymer (HPMA) has significantly reduced the DOX associated cardiotoxicity. However, the reports on the impact of HPMADOX conjugates on the cardiovascular system such as blood pressure (BP) and heart rate (HR) were in restrained animals using tail cuff and/or other methods that lacked the resolution and sensitivity. Herein, we employed radiotelemetric-spectral-echocardiography approach to further understand the in vivo cardiovascular hemodynamics and variability post administration of free DOX and HPMA-DOX. Rats implanted with radiotelemetry device were administered intravenously with DOX (5 mg/kg), HPMA-DOX (5 mg DOX equivalent/kg) and HPMA copolymer and subjected to continuous cardiovascular monitoring and echocardiography for 140 days. We found that DOX-treated rats had ruffled fur, reduced body weight and a low survival rate. Although BP and HR were normal, spectral analysis indicated that their BP and HR variabilities were reduced. All rats exhibited typical signs of cardiotoxicity at histopathology. In contrast, HPMA-DOX rats gained weight over time and survived. Although BP, HR and related variabilities were unaffected, the left ventricular end diastolic volume of these rats, as well as of the HPMA copolymer-treated rats, was found increased at the end of observation period. Additionally, HPMA copolymer caused microscopic injury of the heart tissue. All of these suggest the necessity of caution when employing HPMA as carrier for prolonged drug delivery. The current study also indicates the potential of radiotelemetric-spectral-echocardiography approach for improved preclinical cardiovascular risk assessment of polymer-drug conjugate and other nano-sized-drug constructs.
Requejo-Aguilar, R., Alastrue-Agudo, A., Cases-Villar, M., Lopez-Mocholi, E., England, R., Vicent, M.J.*, and Moreno-Manzano, V., Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials, 2017. 113: p. 18-30. [PubMed]
Spinal cord injury (SCI) suffers from a lack of effective therapeutic strategies. Animal models of acute SCI have provided evidence that transplantation of ependymal stem/progenitor cells of the spinal cord (epSPCs) induces functional recovery, while systemic administration of the anti-inflammatory curcumin provides neuroprotection. However, functional recovery from chronic stage SCI requires additional enhancements in available therapeutic strategies. Herein, we report on a combination treatment for SCI using epSPCs and a pH-responsive polymer-curcumin conjugate. The incorporation of curcumin in a pH-responsive polymeric carrier mainchain, a polyacetal (PA), enhances blood bioavailability, stability, and provides a means for highly localized delivery. We find that PA-curcumin enhances neuroprotection, increases axonal growth, and can improve functional recovery in acute SCI. However, when combined with epSPCs, PA-curcumin also enhances functional recovery in a rodent model of chronic SCI. This suggests that combination therapy may be an exciting new therapeutic option for the treatment of chronic SCI in humans.
Roncador, A., Oppici, E., Talelli, M., Pariente, A.N., Donini, M., Dusi, S., Voltattorni, C.B., Vicent, M.J.*, and Cellini, B., Use of polymer conjugates for the intraperoxisomal delivery of engineered human alanine:glyoxylate aminotransferase as a protein therapy for primary hyperoxaluria type I. Nanomedicine, 2017. 13(3): p. 897-907. [PubMed] [Editorial Comment in J. Urol]
 Alanine:glyoxylate aminotransferase (AGT) is a liver peroxisomal enzyme whose deficit causes the rare disorder Primary Hyperoxaluria Type I (PH1). We now describe the conjugation of poly(ethylene glycol)-co-poly(L-glutamic acid) (PEG-PGA) block-co-polymer to AGT via the formation of disulfide bonds between the polymer and solvent-exposed cysteine residues of the enzyme. PEG-PGA conjugation did not affect AGT structural/functional properties and allowed the enzyme to be internalized in a cellular model of PH1 and to restore glyoxylate-detoxification. The insertion of the C387S/K390S amino acid substitutions, known to favor interaction with the peroxisomal import machinery, reduced conjugation efficiency, but endowed conjugates with the ability to reach the peroxisomal compartment. These results, along with the finding that conjugates are hemocompatible, stable in plasma, and non-immunogenic, hold promise for the development of polypeptide-based AGT conjugates as a therapeutic option for PH1 patients and represent the base for applications to other diseases related to deficits in peroxisomal proteins.
Alanine:glyoxylate aminotransferase (AGT) is a liver peroxisomal enzyme whose deficit causes the rare disorder Primary Hyperoxaluria Type I (PH1). We now describe the conjugation of poly(ethylene glycol)-co-poly(L-glutamic acid) (PEG-PGA) block-co-polymer to AGT via the formation of disulfide bonds between the polymer and solvent-exposed cysteine residues of the enzyme. PEG-PGA conjugation did not affect AGT structural/functional properties and allowed the enzyme to be internalized in a cellular model of PH1 and to restore glyoxylate-detoxification. The insertion of the C387S/K390S amino acid substitutions, known to favor interaction with the peroxisomal import machinery, reduced conjugation efficiency, but endowed conjugates with the ability to reach the peroxisomal compartment. These results, along with the finding that conjugates are hemocompatible, stable in plasma, and non-immunogenic, hold promise for the development of polypeptide-based AGT conjugates as a therapeutic option for PH1 patients and represent the base for applications to other diseases related to deficits in peroxisomal proteins.
Duro-Castano, A., England, R.M., Razola, D., Romero, E., Oteo-Vives, M., Morcillo, M.A., and Vicent, M.J.*, Well-Defined Star-Shaped Polyglutamates with Improved Pharmacokinetic Profiles As Excellent Candidates for Biomedical Applications. Mol Pharm, 2015. 12(10): p. 3639-49. [PubMed]
 There is a need to develop new and innovative polymer carriers to be used as drug delivery systems and/or imaging agents owing to the fact that there is no universal polymeric system that can be used in the treatment of all diseases. Additionally, limitations with existing systems, such as a lack of biodegradability and biocompatibility, inevitably lead to side effects and poor patient compliance. New polymer therapeutics based on amino acids are excellent candidates for drug delivery, as they do not suffer from these limitations. This article reports on a simple yet powerful methodology for the synthesis of 3-arm star-shaped polyglutamic acid with well-defined structures, precise molecular weights (MW), and low polydispersity (D = <1.3). These were synthesized by ring-opening polymerization (ROP) of N-carboxyanhydrides (NCA) in a divergent method from novel multifunctional initiators. Herein, their exhaustive physicochemical characterization is presented. Furthermore, preliminary in vitro evaluation in selected cell models, and exhaustive in vivo biodistribution and pharmacokinetics, highlighted the advantages of these branched systems when compared with their linear counterparts in terms of cell uptake enhancement and prolonged plasma half-life.
There is a need to develop new and innovative polymer carriers to be used as drug delivery systems and/or imaging agents owing to the fact that there is no universal polymeric system that can be used in the treatment of all diseases. Additionally, limitations with existing systems, such as a lack of biodegradability and biocompatibility, inevitably lead to side effects and poor patient compliance. New polymer therapeutics based on amino acids are excellent candidates for drug delivery, as they do not suffer from these limitations. This article reports on a simple yet powerful methodology for the synthesis of 3-arm star-shaped polyglutamic acid with well-defined structures, precise molecular weights (MW), and low polydispersity (D = <1.3). These were synthesized by ring-opening polymerization (ROP) of N-carboxyanhydrides (NCA) in a divergent method from novel multifunctional initiators. Herein, their exhaustive physicochemical characterization is presented. Furthermore, preliminary in vitro evaluation in selected cell models, and exhaustive in vivo biodistribution and pharmacokinetics, highlighted the advantages of these branched systems when compared with their linear counterparts in terms of cell uptake enhancement and prolonged plasma half-life.
Santamaria, B., Ucero, A.C., Benito-Martin, A., Vicent, M.J., Orzaez, M., Celdran, A., Selgas, R., Ruiz-Ortega, M., and Ortiz, A., Biocompatibility reduces inflammation-induced apoptosis in mesothelial cells exposed to peritoneal dialysis fluid. Blood Purif, 2015. 39(1-3): p. 200-9. [PubMed]
BACKGROUND/AIMS: Peritonitis is a major complication that arises out of peritoneal dialysis (PD), leading to death and loss of mesothelium and peritoneal injury, which may impede PD. We studied the combined impact of inflammatory mediators and PD fluids on mesothelial cell death. METHODS: Cultured human mesothelial cells. RESULTS: Inflammatory cytokines (TNF-alpha and interferon-gamma) cooperate with bioincompatible PD fluids containing high glucose degradation product (GDP) concentrations to promote mesothelial cell death. Thus, the inflammatory cytokine cocktail induced a higher rate of death in cells cultured in high GDP PD fluid than in low GDP PD fluid or cell culture medium (cell death expressed as % hypodiploid cells: TNF-alpha and interferon-gamma in RPMI: 14.15 +/- 1.68, TNF-alpha and interferon-gamma in 4.25% low GDP PD fluid 13.16 +/- 3.29, TNF-alpha and interferon-gamma in 4.25% high GDP PD fluid 25.88 +/- 2.18%, p < 0.05 vs. the other two groups). BclxL BH4 peptides, Apaf-1 inhibition or caspase inhibition failed to protect from apoptosis induced by the combination of inflammatory cytokines and bioincompatible PD fluids, although they protected from other forms of mesothelial cell apoptosis. CONCLUSION: Inflammation cooperates with high GDP PD fluids to promote mesothelial cell death, which is resistant to several therapeutic approaches. This information provides a framework for selection of PD fluid during peritonitis.
Gallon, E., Matini, T., Sasso, L., Mantovani, G., Arminan de Benito, A., Sanchis, J., Caliceti, P., Alexander, C., Vicent, M.J., and Salmaso, S., Triblock Copolymer Nanovesicles for pH-Responsive Targeted Delivery and Controlled Release of siRNA to Cancer Cells. Biomacromolecules, 2015. 16(7): p. 1924-37. [PubMed]
 New pH-responsive polymersomes for active anticancer oligonucleotide delivery were prepared from triblock copolymers. The delivery systems were formed by two terminal hydrophilic blocks, PEG and polyglycerolmethacrylate (poly-GMA), and a central weakly basic block, polyimidazole-hexyl methacrylate (poly-ImHeMA), which can complex with oligonucleotides and control vesicle formation/disassembly via pH variations. Targeted polymersomes were prepared by mixing folate-derivatized and underivatized copolymers. At pH 5, ds-DNA was found to complex with the pH-responsive copolymers at a N/P molar ratio above approximately 2:1, which assisted the encapsulation of ds-DNA in the polymersomes, while low association was observed at pH 7.4. Cytotoxicity studies performed on folate receptor overexpressing KB and B16-F10 cells and low folate receptor expressing MCF-7 cells showed high tolerance of the polymersomes at up to 3 mg/mL concentration. Studies performed with red blood cells showed that at pH 5.0 the polymersomes have endosomolytic properties. Cytofluorimetric studies showed a 5.5-fold higher uptake of ds-DNA loaded folate-functional polymersomes in KB cells compared to nontargeted polymersomes. In addition, ds-DNA was found to be localized both in the nucleus and in the cytosol. The incubation of luciferase transfected B16-F10 cells with targeted polymersomes loaded with luciferase and Hsp90 expression silencing siRNAs yielded 31 and 23% knockdown in target protein expression, respectively.
New pH-responsive polymersomes for active anticancer oligonucleotide delivery were prepared from triblock copolymers. The delivery systems were formed by two terminal hydrophilic blocks, PEG and polyglycerolmethacrylate (poly-GMA), and a central weakly basic block, polyimidazole-hexyl methacrylate (poly-ImHeMA), which can complex with oligonucleotides and control vesicle formation/disassembly via pH variations. Targeted polymersomes were prepared by mixing folate-derivatized and underivatized copolymers. At pH 5, ds-DNA was found to complex with the pH-responsive copolymers at a N/P molar ratio above approximately 2:1, which assisted the encapsulation of ds-DNA in the polymersomes, while low association was observed at pH 7.4. Cytotoxicity studies performed on folate receptor overexpressing KB and B16-F10 cells and low folate receptor expressing MCF-7 cells showed high tolerance of the polymersomes at up to 3 mg/mL concentration. Studies performed with red blood cells showed that at pH 5.0 the polymersomes have endosomolytic properties. Cytofluorimetric studies showed a 5.5-fold higher uptake of ds-DNA loaded folate-functional polymersomes in KB cells compared to nontargeted polymersomes. In addition, ds-DNA was found to be localized both in the nucleus and in the cytosol. The incubation of luciferase transfected B16-F10 cells with targeted polymersomes loaded with luciferase and Hsp90 expression silencing siRNAs yielded 31 and 23% knockdown in target protein expression, respectively.
Conejos-Sanchez, I., Cardoso, I., Oteo-Vives, M., Romero-Sanz, E., Paul, A., Sauri, A.R., Morcillo, M.A., Saraiva, M.J., and Vicent, M.J.*, Polymer-doxycycline conjugates as fibril disrupters: an approach towards the treatment of a rare amyloidotic disease. J Control Release, 2015. 198: p. 80-90. [PubMed]
 The term amyloidosis describes neurological diseases where an abnormal protein is misfolded and accumulated as deposits in organs and tissues, known as amyloid, disrupting their normal function. In the most common familial amyloid polyneuropathy (FAP), transthyretin (TTR) displays this role primarily affecting the peripheral nervous system (PNS). Advanced stages of this inherited rare amyloidosis, present as fibril deposits that are responsible for disease progression. In order to stop disease progression, herein we designed an efficient family of nanoconjugates as fibril disrupters. These polymer conjugates are based on doxycycline (doxy), already in phase II trials for Alzheimer’s disease, covalently linked to poly-l-glutamic acid (PGA). The conjugates were rationally designed, looking at drug loading and drug release rate by adequate linker design, always considering the physiological conditions at the molecular target site. Conjugation of doxycycline exhibited greater potential towards TTR fibril disaggregation in vitro compared to the parent drug. Exhaustive physico-chemical evaluation of these polymer-drug conjugates concluded that drug release was unnecessary for activity, highlighting the importance of an appropriate linker. Furthermore, biodistribution studies through optical imaging (OI) and the use of radiolabelled polymer-drug conjugates demonstrated conjugate safety profile and renal clearance route of the selected PGA-doxy candidate, settling the adequacy of our conjugate for future in vivo evaluation. Furthermore, preliminary studies in an FAP in vivo model at early stages of disease development showed non-organ toxicity evidences. This nanosized-system raises a promising treatment for advanced stages of this rare amyloidotic disease, and also presents a starting point for possible application within other amyloidosis-related diseases, such as Alzheimer’s disease.
The term amyloidosis describes neurological diseases where an abnormal protein is misfolded and accumulated as deposits in organs and tissues, known as amyloid, disrupting their normal function. In the most common familial amyloid polyneuropathy (FAP), transthyretin (TTR) displays this role primarily affecting the peripheral nervous system (PNS). Advanced stages of this inherited rare amyloidosis, present as fibril deposits that are responsible for disease progression. In order to stop disease progression, herein we designed an efficient family of nanoconjugates as fibril disrupters. These polymer conjugates are based on doxycycline (doxy), already in phase II trials for Alzheimer’s disease, covalently linked to poly-l-glutamic acid (PGA). The conjugates were rationally designed, looking at drug loading and drug release rate by adequate linker design, always considering the physiological conditions at the molecular target site. Conjugation of doxycycline exhibited greater potential towards TTR fibril disaggregation in vitro compared to the parent drug. Exhaustive physico-chemical evaluation of these polymer-drug conjugates concluded that drug release was unnecessary for activity, highlighting the importance of an appropriate linker. Furthermore, biodistribution studies through optical imaging (OI) and the use of radiolabelled polymer-drug conjugates demonstrated conjugate safety profile and renal clearance route of the selected PGA-doxy candidate, settling the adequacy of our conjugate for future in vivo evaluation. Furthermore, preliminary studies in an FAP in vivo model at early stages of disease development showed non-organ toxicity evidences. This nanosized-system raises a promising treatment for advanced stages of this rare amyloidotic disease, and also presents a starting point for possible application within other amyloidosis-related diseases, such as Alzheimer’s disease.
Casanova-Salas, I., Masia, E., Arminan, A., Calatrava, A., Mancarella, C., Rubio-Briones, J., Scotlandi, K., Vicent, M.J.*, and Lopez-Guerrero, J.A., MiR-187 Targets the Androgen-Regulated Gene ALDH1A3 in Prostate Cancer. PLoS One, 2015. 10(5): p. e0125576. [PubMed]
 miRNAs are predicted to control the activity of approximately 60% of all protein-coding genes participating in the regulation of several cellular processes and diseases, including cancer. Recently, we have demonstrated that miR-187 is significantly downregulated in prostate cancer (PCa) and here we propose a proteomic approach to identify its potential targets. For this purpose, PC-3 cells were transiently transfected with miR-187 precursor and miRNA mimic negative control. Proteins were analyzed by a two-dimensional difference gel electrophoresis (2D-DIGE) and defined as differentially regulated if the observed fold change was +/-1.06. Then, MALDI-TOF MS analysis was performed after protein digestion and low abundance proteins were identified by LC-MS/MS. Peptides were identified by searching against the Expasy SWISS PROT database, and target validation was performed both in vitro by western blot and qRT-PCR and in clinical samples by qRT-PCR, immunohistochemistry and ELISA. DIGE analysis showed 9 differentially expressed spots (p<0.05) and 7 showed a down-regulated expression upon miR-187 re-introduction. Among these targets we identified aldehyde dehydrogenase 1A3 (ALDH1A3). ALDH1A3 expression was significantly downregulated in PC3, LNCaP and DU-145 cells after miR-187 re-introduction. Supporting these data, the expression of ALDH1A3 was found significantly (p<0.0001) up-regulated in PCa samples and inversely correlated (p<0.0001) with miR-187 expression, its expression being directly associated with Gleason score (p = 0.05). The expression of ALDH1A3 was measured in urine samples to evaluate the predictive capability of this biomarker for the presence of PCa and, at a signification level of 10%, PSA and also ALDH1A3 were significantly associated with a positive biopsy of PCa. In conclusion, our data illustrate for the first time the role of ALDH1A3 as a miR-187 target in PCa and provide insights in the utility of using this protein as a new biomarker for PCa.
miRNAs are predicted to control the activity of approximately 60% of all protein-coding genes participating in the regulation of several cellular processes and diseases, including cancer. Recently, we have demonstrated that miR-187 is significantly downregulated in prostate cancer (PCa) and here we propose a proteomic approach to identify its potential targets. For this purpose, PC-3 cells were transiently transfected with miR-187 precursor and miRNA mimic negative control. Proteins were analyzed by a two-dimensional difference gel electrophoresis (2D-DIGE) and defined as differentially regulated if the observed fold change was +/-1.06. Then, MALDI-TOF MS analysis was performed after protein digestion and low abundance proteins were identified by LC-MS/MS. Peptides were identified by searching against the Expasy SWISS PROT database, and target validation was performed both in vitro by western blot and qRT-PCR and in clinical samples by qRT-PCR, immunohistochemistry and ELISA. DIGE analysis showed 9 differentially expressed spots (p<0.05) and 7 showed a down-regulated expression upon miR-187 re-introduction. Among these targets we identified aldehyde dehydrogenase 1A3 (ALDH1A3). ALDH1A3 expression was significantly downregulated in PC3, LNCaP and DU-145 cells after miR-187 re-introduction. Supporting these data, the expression of ALDH1A3 was found significantly (p<0.0001) up-regulated in PCa samples and inversely correlated (p<0.0001) with miR-187 expression, its expression being directly associated with Gleason score (p = 0.05). The expression of ALDH1A3 was measured in urine samples to evaluate the predictive capability of this biomarker for the presence of PCa and, at a signification level of 10%, PSA and also ALDH1A3 were significantly associated with a positive biopsy of PCa. In conclusion, our data illustrate for the first time the role of ALDH1A3 as a miR-187 target in PCa and provide insights in the utility of using this protein as a new biomarker for PCa.
Talelli, M. and Vicent, M.J.*, Reduction sensitive Poly(l-glutamic acid) (PGA)-protein conjugates designed for polymer masked-unmasked protein therapy. Biomacromolecules, 2014. 15(11): p. 4168-77. [PubMed]
 Protein therapeutics have become an important class of medicines for a large variety of diseases. However, they have disadvantages such as rapid elimination/metabolism leading to the need for repeated doses, immunogenicity/antigenicity, and aggregation/degradation during formulation and storage. The concept of polymer masked-unmasked protein therapy (PUMPT) makes use of polymer-protein multivalent conjugation with biodegradable carriers, which mask the protein activity during transport and increase its stability, but is capable of specifically triggering an unmasking effect at the disease site, allowing its therapeutic action. The aim of this study was to widen the PUMPT concept by designing reduction sensitive poly-l-glutamic acid (PGA)-based conjugates, in which the protein release and unmasking effect takes place in the reducing environments found intracellularly as well as in the tumor microenvironment. Lysozyme was used as the model protein to achieve proof of concept. Overall, the synthesized platform showed to be promising for the delivery of anticancer proteins as well as for enzyme replacement therapeutic approaches aiming to treat lysosomal storage disorders.
Protein therapeutics have become an important class of medicines for a large variety of diseases. However, they have disadvantages such as rapid elimination/metabolism leading to the need for repeated doses, immunogenicity/antigenicity, and aggregation/degradation during formulation and storage. The concept of polymer masked-unmasked protein therapy (PUMPT) makes use of polymer-protein multivalent conjugation with biodegradable carriers, which mask the protein activity during transport and increase its stability, but is capable of specifically triggering an unmasking effect at the disease site, allowing its therapeutic action. The aim of this study was to widen the PUMPT concept by designing reduction sensitive poly-l-glutamic acid (PGA)-based conjugates, in which the protein release and unmasking effect takes place in the reducing environments found intracellularly as well as in the tumor microenvironment. Lysozyme was used as the model protein to achieve proof of concept. Overall, the synthesized platform showed to be promising for the delivery of anticancer proteins as well as for enzyme replacement therapeutic approaches aiming to treat lysosomal storage disorders.
Matini, T., Francini, N., Battocchio, A., Spain, S.G., Mantovani, G., Vicent, M.J.*, Sanchis, J., Gallon, E., Mastrotto, F., Salmaso, S., Caliceti, P., and Alexander, C., Synthesis and characterization of variable conformation pH responsive block co-polymers for nucleic acid delivery and targeted cell entry. Polymer Chemistry, 2014. 5(5): p. 1626-1636. [Link]
 Responsive materials that change conformation with varying pH have been prepared from a range of amphiphilic block co-polymers. The individual blocks are composed of (a) permanently hydrophilic chains with neutral functionality and (b) acrylate polymers with weakly basic side-chains. Variation in co-monomer content, molar mass and block ratios/compositions leads to a range of pH-responses, manifest through reversible self-assembly into micelles and/or polymersomes. These transitions can be tuned to achieve environmental responses in a pH range from 5-7, as shown by turbidimetric analysis, NMR and dynamic light scattering measurements (DLS). Further characterization by transmission electron microscopy (TEM) indicates that polymersomes with diameters of 100-200 nm can be formed under certain pH-ranges where the weakly basic side-chains are deprotonated. The ability of the systems assembled with these polymers to act as pH-responsive containers is shown by DNA encapsulation and release studies, and their potential for application as vehicle for drug delivery is proved by cell metabolic activity and cell uptake measurements.
Responsive materials that change conformation with varying pH have been prepared from a range of amphiphilic block co-polymers. The individual blocks are composed of (a) permanently hydrophilic chains with neutral functionality and (b) acrylate polymers with weakly basic side-chains. Variation in co-monomer content, molar mass and block ratios/compositions leads to a range of pH-responses, manifest through reversible self-assembly into micelles and/or polymersomes. These transitions can be tuned to achieve environmental responses in a pH range from 5-7, as shown by turbidimetric analysis, NMR and dynamic light scattering measurements (DLS). Further characterization by transmission electron microscopy (TEM) indicates that polymersomes with diameters of 100-200 nm can be formed under certain pH-ranges where the weakly basic side-chains are deprotonated. The ability of the systems assembled with these polymers to act as pH-responsive containers is shown by DNA encapsulation and release studies, and their potential for application as vehicle for drug delivery is proved by cell metabolic activity and cell uptake measurements.
Conejos-Sanchez, I., Cardoso, I., Saraiva, M.J., and Vicent, M.J.*, Targeting a rare amyloidotic disease through rationally designed polymer conjugates. J Control Release, 2014. 178: p. 95-100. [PubMed]
 Saraiva et al. discovered in 2006 a RAGE-based peptide sequence capable of preventing transthyretin (TTR) aggregate-induced cytotoxicity, hallmark of initial stages of an inherited rare amyloidosis known as Familial Amyloidotic Polyneuropathy (FAP). To allow clinical progression of this peptidic sequence as FAP treatment, a family of polymer conjugates has been designed, synthesised and fully characterised. This approach fulfils the strategies defined in the Polymer Therapeutics area as an exhaustive physico-chemical characterisation fitting activity output towards a novel molecular target that is described here. RAGE peptide acts extracellularly, therefore, no intracellular drug delivery was necessary. PEG was selected as carrier and polymer-drug linker optimisation was then carried out by means of biodegradable (disulphide) and non-biodegradable (amide) covalent bonds. Conjugate size in solution, stability under in vitro and in vivo scenarios and TTR binding affinity through surface plasmon resonance (SPR) was also performed with all synthesised conjugates. In their in vitro evaluation by monitoring the activation of caspase-3 in Schwann cells, peptide derivatives demonstrated retention of peptide activity reducing TTR aggregates (TTRagg) cytotoxicity upon conjugation and a greater plasma stability than the parent free peptide. The results also confirmed that a more stable polymer-peptide linker (amide) is required to secure therapeutic efficiency.
Saraiva et al. discovered in 2006 a RAGE-based peptide sequence capable of preventing transthyretin (TTR) aggregate-induced cytotoxicity, hallmark of initial stages of an inherited rare amyloidosis known as Familial Amyloidotic Polyneuropathy (FAP). To allow clinical progression of this peptidic sequence as FAP treatment, a family of polymer conjugates has been designed, synthesised and fully characterised. This approach fulfils the strategies defined in the Polymer Therapeutics area as an exhaustive physico-chemical characterisation fitting activity output towards a novel molecular target that is described here. RAGE peptide acts extracellularly, therefore, no intracellular drug delivery was necessary. PEG was selected as carrier and polymer-drug linker optimisation was then carried out by means of biodegradable (disulphide) and non-biodegradable (amide) covalent bonds. Conjugate size in solution, stability under in vitro and in vivo scenarios and TTR binding affinity through surface plasmon resonance (SPR) was also performed with all synthesised conjugates. In their in vitro evaluation by monitoring the activation of caspase-3 in Schwann cells, peptide derivatives demonstrated retention of peptide activity reducing TTR aggregates (TTRagg) cytotoxicity upon conjugation and a greater plasma stability than the parent free peptide. The results also confirmed that a more stable polymer-peptide linker (amide) is required to secure therapeutic efficiency.
Casanova-Salas, I., Rubio-Briones, J., Calatrava, A., Mancarella, C., Masia, E., Casanova, J., Fernandez-Serra, A., Rubio, L., Ramirez-Backhaus, M., Arminan, A., Dominguez-Escrig, J., Martinez, F., Garcia-Casado, Z., Scotlandi, K., Vicent, M.J., and Lopez-Guerrero, J.A., Identification of miR-187 and miR-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J Urol, 2014. 192(1): p. 252-9. [PubMed]
PURPOSE: miRNAs are noncoding RNAs that negatively regulate target mRNA gene expression. Aberrant miRNA expression is associated with prostate cancer pathogenesis. We identified miRNAs as potential biomarkers for prostate cancer diagnosis and prognosis. MATERIALS AND METHODS: Total RNA was obtained from 10 normal prostate and 50 prostate cancer samples, and analyzed using the GeneChip(R) miRNA 2.0 Array. At a median followup of 92 months (range 2 to 189) an independent cohort of 273 paraffin embedded prostate cancer samples was used for validation by quantitative reverse transcriptase-polymerase chain reaction. Another 92 urine samples from patients undergoing prostate biopsy were evaluated for these miRNAs. RESULTS: miR-182 and 187, the miRNAs most differentially expressed between normal and tumor tissue, were selected for further validation. miR-187 inversely correlated with cT (p = 0.125) and pT (p = 0.0002) stages, Gleason score (p = 0.003) and TMPRSS2-ERG status (p = 0.003). The log rank test showed associations of miR-182 with biochemical (p = 0.026) and clinical (p = 0.043) progression-free survival, as also noted on multivariate analysis. A significant independent improvement in the definition of risk of progression was achieved by combining miR-182 expression with Gleason score (p <0.0001). miR-187 detection in urine provided an independent predictive value for positive biopsy. A prediction model including serum prostate specific antigen, urine PCA3 and miR-187 provided 88.6% sensitivity and 50% specificity (AUC 0.711, p = 0.001). CONCLUSIONS: Results show that miR-182 and 187 are promising biomarkers for prostate cancer prognosis to identify patients at risk for progression and for diagnosis to improve the predictive capability of existing biomarkers.
Ucero, A.C., Berzal, S., Ocana-Salceda, C., Sancho, M., Orzaez, M., Messeguer, A., Ruiz-Ortega, M., Egido, J., Vicent, M.J., Ortiz, A., and Ramos, A.M., A polymeric nanomedicine diminishes inflammatory events in renal tubular cells. PLoS One, 2013. 8(1): p. e51992. [PubMed]
The polyglutamic acid/peptoid 1 (QM56) nanoconjugate inhibits apoptosis by interfering with Apaf-1 binding to procaspase-9. We now describe anti-inflammatory properties of QM56 in mouse kidney and renal cell models.In cultured murine tubular cells, QM56 inhibited the inflammatory response to Tweak, a non-apoptotic stimulus. Tweak induced MCP-1 and Rantes synthesis through JAK2 kinase and NF-kappaB activation. Similar to JAK2 kinase inhibitors, QM56 inhibited Tweak-induced NF-kappaB transcriptional activity and chemokine expression, despite failing to inhibit NF-kappaB-p65 nuclear translocation and NF-kappaB DNA binding. QM56 prevented JAK2 activation and NF-kappaB-p65(Ser536) phosphorylation. The anti-inflammatory effect and JAK2 inhibition by QM56 were observed in Apaf-1(-/-) cells. In murine acute kidney injury, QM56 decreased tubular cell apoptosis and kidney inflammation as measured by down-modulations of MCP-1 and Rantes mRNA expression, immune cell infiltration and activation of the JAK2-dependent inflammatory pathway.In conclusion, QM56 has an anti-inflammatory activity which is independent from its role as inhibitor of Apaf-1 and apoptosis and may have potential therapeutic relevance.
Conejos-Sanchez, I., Duro-Castano, A., Birke, A., Barz, M., and Vicent, M.J.*, A controlled and versatile NCA polymerization method for the synthesis of polypeptides. Polymer Chemistry, 2013. 4(11): p. 3182-3186. [Link]
 A versatile and simple methodology for the preparation of well-defined polyglutamate nanocarriers is described. For the first time ammonium salts with non-nucleophilic tetrafluoroborate anions are used as initiators for the ring opening polymerization of [small alpha]-N-carboxyanhydrides (NCAs) allowing a multigram scale polyglutamate synthesis with defined molecular weight (up to 800 units), low polydispersity (<1.2), controlled chain end functionality and adequate stereoselectivity and absence of any trace of toxic impurity to allow biomedical applications.
A versatile and simple methodology for the preparation of well-defined polyglutamate nanocarriers is described. For the first time ammonium salts with non-nucleophilic tetrafluoroborate anions are used as initiators for the ring opening polymerization of [small alpha]-N-carboxyanhydrides (NCAs) allowing a multigram scale polyglutamate synthesis with defined molecular weight (up to 800 units), low polydispersity (<1.2), controlled chain end functionality and adequate stereoselectivity and absence of any trace of toxic impurity to allow biomedical applications.
Barz, M., Duro-Castano, A., and Vicent, M.J.*, A versatile post-polymerization modification method for polyglutamic acid: synthesis of orthogonal reactive polyglutamates and their use in “click chemistry”. Polymer Chemistry, 2013. 4(10): p. 2989-2994. [Link]
 In this article we describe a versatile methodology for the synthesis of polyglutamic acid (PGA) derivatives bearing orthogonal reactive sites. The reactive groups enable selective conjugation chemistry by copper catalyzed azide-alkyne coupling (CuAAC). PGA was derived in aqueous media as well as in organic media using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl morpholinium chloride (DMTTM) salts. The spectra of attached chemical moieties ranges from simple PEGylation with 2,5,8,11,14,17,20-heptaoxadocosan-22-amine (mEG(6)NH2) to the incorporation of propargylamine, 11-azido-3,6,9-trioxaundecan-1-amine (NH2-EG(2)N3), and 20-azido-3,6,9,12,15,18-hexaoxaicosan-1-amine (NH2-EG(6)N3). Herein, it is demonstrated that the degree of functionalization can be easily controlled within this one pot reaction. Additionally, we report conditions for the CuAAC with various PGA derivatives, which can be employed for site-specific conjugation of either hydrophilic or hydrophobic compounds.
In this article we describe a versatile methodology for the synthesis of polyglutamic acid (PGA) derivatives bearing orthogonal reactive sites. The reactive groups enable selective conjugation chemistry by copper catalyzed azide-alkyne coupling (CuAAC). PGA was derived in aqueous media as well as in organic media using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl morpholinium chloride (DMTTM) salts. The spectra of attached chemical moieties ranges from simple PEGylation with 2,5,8,11,14,17,20-heptaoxadocosan-22-amine (mEG(6)NH2) to the incorporation of propargylamine, 11-azido-3,6,9-trioxaundecan-1-amine (NH2-EG(2)N3), and 20-azido-3,6,9,12,15,18-hexaoxaicosan-1-amine (NH2-EG(6)N3). Herein, it is demonstrated that the degree of functionalization can be easily controlled within this one pot reaction. Additionally, we report conditions for the CuAAC with various PGA derivatives, which can be employed for site-specific conjugation of either hydrophilic or hydrophobic compounds.
Gimenez, V., James, C., Arminan, A., Schweins, R., Paul, A., and Vicent, M.J.*, Demonstrating the importance of polymer-conjugate conformation in solution on its therapeutic output: Diethylstilbestrol (DES)-polyacetals as prostate cancer treatment. J Control Release, 2012. 159(2): p. 290-301. [PubMed]
 The design of improved polymeric carriers to be used in the next generation of polymer therapeutics is an ongoing challenge. Biodegradable systems present potential advantages regarding safety benefit apart from the possibility to use higher molecular weight (Mw) carriers allowing PK optimization, by exploiting the enhanced permeability and retention (EPR)-mediated tumor targeting. Within this context, we previously designed pH-responsive polyacetalic systems, tert-polymers, where a drug with the adequate diol-functionality was incorporated within the polymer mainchain. The synthetic, non-steroidal estrogen, diethylstilboestrol (DES) clinically used for the treatment of advanced prostate cancer was chosen as drug. In order to improve the properties of this tert-polymer, novel polyacetalic systems as block-co-polymers, with more defined structure have been obtained. This second generation polyacetals allowed higher drug capacity than the tert-polymer, a biphasic DES release profile at acidic pH and due to its controlled amphiphilic character readily formed micelle-like structures in solution. These features result in an enhancement of conjugate therapeutic value in selected prostate cancer cell models. Exhaustive physico-chemical characterization focusing on nanoconjugate solution behavior and using advanced techniques, such as, pulsed-gradient spin-echo NMR (PGSE-NMR) and small-angle neutron scattering (SANS), has been carried out in order to demonstrate this hypothesis. Clear evidence of significantly different conformation in solution has been obtained for both polyacetals. These results demonstrate that an adequate control on molecular or supramolecular conformation in solution with polymer therapeutics is crucial in order to achieve the desired therapeutic output.
The design of improved polymeric carriers to be used in the next generation of polymer therapeutics is an ongoing challenge. Biodegradable systems present potential advantages regarding safety benefit apart from the possibility to use higher molecular weight (Mw) carriers allowing PK optimization, by exploiting the enhanced permeability and retention (EPR)-mediated tumor targeting. Within this context, we previously designed pH-responsive polyacetalic systems, tert-polymers, where a drug with the adequate diol-functionality was incorporated within the polymer mainchain. The synthetic, non-steroidal estrogen, diethylstilboestrol (DES) clinically used for the treatment of advanced prostate cancer was chosen as drug. In order to improve the properties of this tert-polymer, novel polyacetalic systems as block-co-polymers, with more defined structure have been obtained. This second generation polyacetals allowed higher drug capacity than the tert-polymer, a biphasic DES release profile at acidic pH and due to its controlled amphiphilic character readily formed micelle-like structures in solution. These features result in an enhancement of conjugate therapeutic value in selected prostate cancer cell models. Exhaustive physico-chemical characterization focusing on nanoconjugate solution behavior and using advanced techniques, such as, pulsed-gradient spin-echo NMR (PGSE-NMR) and small-angle neutron scattering (SANS), has been carried out in order to demonstrate this hypothesis. Clear evidence of significantly different conformation in solution has been obtained for both polyacetals. These results demonstrate that an adequate control on molecular or supramolecular conformation in solution with polymer therapeutics is crucial in order to achieve the desired therapeutic output.
England, R.M., Masia, E., Gimenez, V., Lucas, R., and Vicent, M.J.*, Polyacetal-stilbene conjugates – The first examples of polymer therapeutics for the inhibition of HIF-1 in the treatment of solid tumours. J Control Release, 2012. 164(3): p. 314-22. [PubMed]
 We report here the first examples of Polymer Therapeutics synthesised with the intention of inhibiting Hypoxia Inducible Factor-1 (HIF-1), a transcription factor heavily involved in numerous cell processes under a low oxygen environment. Four compounds were selected for use in these systems; Diethylstilbestrol (DES), Bisphenol A (BIS), Dienestrol (DIENES) and Hexestrol (HEX), which were chosen from a large family of similar molecules known as Stilbenes. These are non-steroidal molecules with structural similarities to oestrogen, and of which DES and BIS have previously been reported for HIF-1 inhibition. These molecules were incorporated into a poly(ethylene glycol) (PEG) based polyacetal system using a reaction of short PEG chains with di(ethylene glycol) divinyl ether units and an acid catalyst and without the need for biodegradable linkers. With an improved polyacetal synthesis strategy we obtained high yields of water soluble polymer conjugates with desirable drug loadings and tailored molecular weights (Mw 23,000-35,000g/mol) with relatively narrow polydispersities (pdi 1.3-1.5). These polymers were found to be hydrolytically cleaved under acid conditions (such as those found in endosomes, lysosomes or the extracellular fluid of some tumours) yielding the free drug. Additionally, they were found to be stable over prolonged periods of time at pH 7.4 mimicking blood plasma. Of the four polymers synthesised, the conjugates of DES and BIS displayed the best activity for HIF-1alpha inhibition in HeLa 9xHRE-Luc tumour cells. More importantly, these conjugates were found to exhibit little to no cell toxicity, contrary to the free drugs, and consequently, they significantly enhanced drug therapeutic index (TI 3.5 vs. 7.2 for free DES vs. DES-polyacetal 2a, and TI 1.1 vs. >20 for free BIS vs. BIS-polyacetal 1b).
We report here the first examples of Polymer Therapeutics synthesised with the intention of inhibiting Hypoxia Inducible Factor-1 (HIF-1), a transcription factor heavily involved in numerous cell processes under a low oxygen environment. Four compounds were selected for use in these systems; Diethylstilbestrol (DES), Bisphenol A (BIS), Dienestrol (DIENES) and Hexestrol (HEX), which were chosen from a large family of similar molecules known as Stilbenes. These are non-steroidal molecules with structural similarities to oestrogen, and of which DES and BIS have previously been reported for HIF-1 inhibition. These molecules were incorporated into a poly(ethylene glycol) (PEG) based polyacetal system using a reaction of short PEG chains with di(ethylene glycol) divinyl ether units and an acid catalyst and without the need for biodegradable linkers. With an improved polyacetal synthesis strategy we obtained high yields of water soluble polymer conjugates with desirable drug loadings and tailored molecular weights (Mw 23,000-35,000g/mol) with relatively narrow polydispersities (pdi 1.3-1.5). These polymers were found to be hydrolytically cleaved under acid conditions (such as those found in endosomes, lysosomes or the extracellular fluid of some tumours) yielding the free drug. Additionally, they were found to be stable over prolonged periods of time at pH 7.4 mimicking blood plasma. Of the four polymers synthesised, the conjugates of DES and BIS displayed the best activity for HIF-1alpha inhibition in HeLa 9xHRE-Luc tumour cells. More importantly, these conjugates were found to exhibit little to no cell toxicity, contrary to the free drugs, and consequently, they significantly enhanced drug therapeutic index (TI 3.5 vs. 7.2 for free DES vs. DES-polyacetal 2a, and TI 1.1 vs. >20 for free BIS vs. BIS-polyacetal 1b).
Barz, M., Arminan, A., Canal, F., Wolf, F., Koynov, K., Frey, H., Zentel, R., and Vicent, M.J.*, P(HPMA)-block-P(LA) copolymers in paclitaxel formulations: polylactide stereochemistry controls micellization, cellular uptake kinetics, intracellular localization and drug efficiency. J Control Release, 2012. 163(1): p. 63-74. [PubMed]
 In order to explore the influence of polymer microstructure and stereochemistry in biological settings, the synthesis, micellization, cellular fate and the use in paclitaxel formulations of poly(N-(2-hydroxypropyl)-methacrylamide)-block-poly(L-lactide) (P(HPMA)-block-P(LLA)) and poly(N-(2-hydroxypropyl)-methacrylamide)-block-poly(DL-lactide) block copolymers (P(HPMA)-block-P(DLLA)) were studied. To this end, P(HPMA)-block-P(lactide) block copolymers and their fluorescently labeled analogues were synthesized. The polymers exhibited molecular weights M(n) around 20,000 g/mol with dispersities (D=M(w)/M(n)) below 1.3. In addition, the solution conformation of this new type of partially degradable amphiphilic block copolymers was studied with and without paclitaxel loading in PBS buffer (pH 7.2), employing fluorescence correlation spectroscopy (FCS). We observed polymeric micelles with a hydrodynamic diameter of 17.0 nm for a fluorescently labeled P(HPMA)-block-P(LLA) block copolymer (P2*) and 20.4 nm for a P(HPMA)-block-P(DLLA) block copolymer (P3*). For the corresponding loaded block copolymers aggregates with a diameter of 40.0 nm (P2*) and 41.4 nm (P3*) in formulations containing 17 wt.% paclitaxel were observed, respectively. While the block copolymer itself showed non-toxic behavior up to a concentration of 3 mg/mL in HeLa (human cervix adenocarcinoma) cells, the paclitaxel containing formulations showed IC 50 values in the range of 10-100 nM. The P(HPMA)-block-P(DLLA) polymer (P3*) enters the cells more efficiently than stereo regular polymer (P2*) via an energy-dependent uptake mechanism. Thus, differences in the IC(50) value are–most likely–attributed to significant changes in cellular uptake. Polymer tacticity and stereoregularity appear to represent a key feature determining cellular uptake and efficiency for the PLA block copolymer drug formulations. This work demonstrates the importance of the microstructure of polymers used in drug delivery systems (DDS).
In order to explore the influence of polymer microstructure and stereochemistry in biological settings, the synthesis, micellization, cellular fate and the use in paclitaxel formulations of poly(N-(2-hydroxypropyl)-methacrylamide)-block-poly(L-lactide) (P(HPMA)-block-P(LLA)) and poly(N-(2-hydroxypropyl)-methacrylamide)-block-poly(DL-lactide) block copolymers (P(HPMA)-block-P(DLLA)) were studied. To this end, P(HPMA)-block-P(lactide) block copolymers and their fluorescently labeled analogues were synthesized. The polymers exhibited molecular weights M(n) around 20,000 g/mol with dispersities (D=M(w)/M(n)) below 1.3. In addition, the solution conformation of this new type of partially degradable amphiphilic block copolymers was studied with and without paclitaxel loading in PBS buffer (pH 7.2), employing fluorescence correlation spectroscopy (FCS). We observed polymeric micelles with a hydrodynamic diameter of 17.0 nm for a fluorescently labeled P(HPMA)-block-P(LLA) block copolymer (P2*) and 20.4 nm for a P(HPMA)-block-P(DLLA) block copolymer (P3*). For the corresponding loaded block copolymers aggregates with a diameter of 40.0 nm (P2*) and 41.4 nm (P3*) in formulations containing 17 wt.% paclitaxel were observed, respectively. While the block copolymer itself showed non-toxic behavior up to a concentration of 3 mg/mL in HeLa (human cervix adenocarcinoma) cells, the paclitaxel containing formulations showed IC 50 values in the range of 10-100 nM. The P(HPMA)-block-P(DLLA) polymer (P3*) enters the cells more efficiently than stereo regular polymer (P2*) via an energy-dependent uptake mechanism. Thus, differences in the IC(50) value are–most likely–attributed to significant changes in cellular uptake. Polymer tacticity and stereoregularity appear to represent a key feature determining cellular uptake and efficiency for the PLA block copolymer drug formulations. This work demonstrates the importance of the microstructure of polymers used in drug delivery systems (DDS).
Eldar-Boock, A., Miller, K., Sanchis, J., Lupu, R., Vicent, M.J.*, and Satchi-Fainaro, R., Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel. Biomaterials, 2011. 32(15): p. 3862-74. [PubMed]
Angiogenesis plays a prominent role in cancer progression. Anti-angiogenic therapy therefore, either alone or in combination with conventional cytotoxic therapy, offers a promising therapeutic approach. Paclitaxel (PTX) is a widely-used potent cytotoxic drug that also exhibits anti-angiogenic effects at low doses. However, its use, at its full potential, is limited by severe side effects. Here we designed and synthesized a targeted conjugate of PTX, a polymer and an integrin-targeted moiety resulting in a polyglutamic acid (PGA)-PTX-E-[c(RGDfK)(2)] nano-scaled conjugate. Polymer conjugation converted PTX to a macromolecule, which passively targets the tumor tissue exploiting the enhanced permeability and retention effect, while extravasating via the leaky tumor neovasculature. The cyclic RGD peptidomimetic enhanced the effects previously seen for PGA-PTX alone, utilizing the additional active targeting to the alpha(v)beta(3) integrin overexpressed on tumor endothelial and epithelial cells. This strategy is particularly valuable when tumors are well-vascularized, but they present poor vascular permeability. We show that PGA is enzymatically-degradable leading to PTX release under lysosomal acidic pH. PGA-PTX-E-[c(RGDfK)(2)] inhibited the growth of proliferating alpha(v)beta(3)-expressing endothelial cells and several cancer cells. We also showed that PGA-PTX-E-[c(RGDfK)(2)] blocked endothelial cells migration towards vascular endothelial growth factor; blocked capillary-like tube formation; and inhibited endothelial cells attachment to fibrinogen. Orthotopic studies in mice demonstrated preferential tumor accumulation of the RGD-bearing conjugate, leading to enhanced anti-tumor efficacy and a marked decrease in toxicity as compared with free PTX-treated mice.
Deacon, S.P., Apostolovic, B., Carbajo, R.J., Schott, A.K., Beck, K., Vicent, M.J., Pineda-Lucena, A., Klok, H.A., and Duncan, R., Polymer coiled-coil conjugates: potential for development as a new class of therapeutic “molecular switch”. Biomacromolecules, 2011. 12(1): p. 19-27. [PubMed]
 Polymer therapeutics, including polymeric drugs and polymer-protein conjugates, are clinically established as first-generation nanomedicines. Knowing that the coiled-coil peptide motif is fundamentally important in the regulation of many cellular and pathological processes, the aim of these studies was to examine the feasibility of designing polymer conjugates containing the coiled-coil motif as a putative therapeutic “molecular switch”. To establish proof of concept, we prepared a mPEG-FosW(C) conjugate by reacting mPEG-maleimide (M(w) 5522 g mol(-1), M(w)/M(n) 1.1) with a FosW peptide synthesized to contain a terminal cysteine residue (FosW(C)). Its ability to form a stable coil-coil heterodimer with the target c-Jun sequence of the oncogenic AP-1 transcription factor was investigated using 2D (15)N-HSQC NMR together with a recombinantly prepared (15)N-labeled c-Jun peptide ([(15)N]r-c-Jun). Observation that heterodimerization was achieved and that the polymer did not sterically disadvantage hybridization suggests an important future for this new family of polymer therapeutics.
Polymer therapeutics, including polymeric drugs and polymer-protein conjugates, are clinically established as first-generation nanomedicines. Knowing that the coiled-coil peptide motif is fundamentally important in the regulation of many cellular and pathological processes, the aim of these studies was to examine the feasibility of designing polymer conjugates containing the coiled-coil motif as a putative therapeutic “molecular switch”. To establish proof of concept, we prepared a mPEG-FosW(C) conjugate by reacting mPEG-maleimide (M(w) 5522 g mol(-1), M(w)/M(n) 1.1) with a FosW peptide synthesized to contain a terminal cysteine residue (FosW(C)). Its ability to form a stable coil-coil heterodimer with the target c-Jun sequence of the oncogenic AP-1 transcription factor was investigated using 2D (15)N-HSQC NMR together with a recombinantly prepared (15)N-labeled c-Jun peptide ([(15)N]r-c-Jun). Observation that heterodimerization was achieved and that the polymer did not sterically disadvantage hybridization suggests an important future for this new family of polymer therapeutics.
Vicent, M.J.*, Cascales, L., Carbajo, R.J., Cortes, N., Messeguer, A., and Perez Paya, E., Nanoconjugates as intracorporeal neutralizers of bacterial endotoxins. J Control Release, 2010. 142(2): p. 277-85. [PubMed]
 Sepsis is a leading cause of mortality that is most often provoked by endotoxins (i.e. lipopolysaccharides; LPS) released by Gram-negative bacteria into the patient’s bloodstream during infection. The therapeutic armory currently available for sepsis treatment is poor. We previously identified an LPS-neutralizing small molecule, PTD7. Here we tested the efficacy of novel PTD7-nanoconjugates in a murine model of sepsis. We found that PTD7-based nanoconjugates treated mice had improved survival that it was correlated with a marked decrease in proinflammatory cytokines in the blood. This proves that nanoconjugate-based endotoxin neutralizers can function as intracorporeal neutralizers of bacterial endotoxins.
Sepsis is a leading cause of mortality that is most often provoked by endotoxins (i.e. lipopolysaccharides; LPS) released by Gram-negative bacteria into the patient’s bloodstream during infection. The therapeutic armory currently available for sepsis treatment is poor. We previously identified an LPS-neutralizing small molecule, PTD7. Here we tested the efficacy of novel PTD7-nanoconjugates in a murine model of sepsis. We found that PTD7-based nanoconjugates treated mice had improved survival that it was correlated with a marked decrease in proinflammatory cytokines in the blood. This proves that nanoconjugate-based endotoxin neutralizers can function as intracorporeal neutralizers of bacterial endotoxins.
Canal, F., Vicent, M.J.*, Pasut, G., and Schiavon, O., Relevance of folic acid/polymer ratio in targeted PEG-epirubicin conjugates. J Control Release, 2010. 146(3): p. 388-99. [PubMed]
 A series of PEG-epirubicin conjugates with different folic acid contents per polymer chain was synthesized in order to study the influence of polymer/targeting moiety ratio on selective cytotoxicity, cellular uptake and intracellular localization. Analogous carboxyl-terminated conjugates without folic acid were studied as control. The heterobifunctional HO-PEG-COOH was used as polymeric carrier, allowing the synthesis of conjugates with a good control over the chemical structure and the drug/polymer and polymer/targeting residue ratios. A dendron structure was synthesized at one end of the PEG chain with the aim to increase the number of folic acid molecules. L-2-aminoadipic acid was used as branching unit. The conjugates showed high stability under several physiological conditions. Biological evaluation was carried out in A549, HeLa and KB-3-1 human cell lines, as these cells have different levels of folate receptor (FR) expression. In particular A549 cells are FR negative (FR-), HeLa cells are FR positive (FR+) and KB-3-1 cells over-express FR (FR++). It was clearly shown that the biological activity of the conjugates was influenced by the presence and the number of folic acid molecules per polymer chain and by the level of FR expression on cell surface. Conjugates conformation in solution was also studied, as differences in size might well affect cell internalization. In the cell viability assay, conjugates without folic acid were unexpectedly more cytotoxic than the targeted conjugates, but their IC(50) values were similar in the three cell lines. Differently, the anti-proliferative activity of targeted derivatives markedly increased going from FR(-) to FR(++) cells. FACS and confocal microscopy studies showed greater cellular internalization with the targeted conjugates than with their non-targeted analogues; more importantly, this relationship is clearly dependent on folic acid content in the conjugates and FR expression level in the cell line used.
A series of PEG-epirubicin conjugates with different folic acid contents per polymer chain was synthesized in order to study the influence of polymer/targeting moiety ratio on selective cytotoxicity, cellular uptake and intracellular localization. Analogous carboxyl-terminated conjugates without folic acid were studied as control. The heterobifunctional HO-PEG-COOH was used as polymeric carrier, allowing the synthesis of conjugates with a good control over the chemical structure and the drug/polymer and polymer/targeting residue ratios. A dendron structure was synthesized at one end of the PEG chain with the aim to increase the number of folic acid molecules. L-2-aminoadipic acid was used as branching unit. The conjugates showed high stability under several physiological conditions. Biological evaluation was carried out in A549, HeLa and KB-3-1 human cell lines, as these cells have different levels of folate receptor (FR) expression. In particular A549 cells are FR negative (FR-), HeLa cells are FR positive (FR+) and KB-3-1 cells over-express FR (FR++). It was clearly shown that the biological activity of the conjugates was influenced by the presence and the number of folic acid molecules per polymer chain and by the level of FR expression on cell surface. Conjugates conformation in solution was also studied, as differences in size might well affect cell internalization. In the cell viability assay, conjugates without folic acid were unexpectedly more cytotoxic than the targeted conjugates, but their IC(50) values were similar in the three cell lines. Differently, the anti-proliferative activity of targeted derivatives markedly increased going from FR(-) to FR(++) cells. FACS and confocal microscopy studies showed greater cellular internalization with the targeted conjugates than with their non-targeted analogues; more importantly, this relationship is clearly dependent on folic acid content in the conjugates and FR expression level in the cell line used.
Barz, M., Wolf, F.K., Canal, F., Koynov, K., Vicent, M.J., Frey, H., and Zentel, R., Synthesis, Characterization and Preliminary Biological Evaluation of P(HPMA)-b-P(LLA) Copolymers: A New Type of Functional Biocompatible Block Copolymer. Macromol Rapid Commun, 2010. 31(17): p. 1492-500. [PubMed]
 We describe a synthetic pathway to functional P(HPMA)-b-P(LLA) block copolymers. The synthesis relies on a combination of ring-opening polymerization of L-lactide, conversion into a chain transfer agent (CTA) for the RAFT polymerization of pentafluorophenyl methacrylate. A series of block copolymers was prepared that exhibited molecular weights $\overline M _{\rm n}$ ranging from 7 600 to 34 300 g . mol(-1) , with moderate PDI between 1.3 and 1.45. These reactive precursor polymers have been transformed into biocompatible P(HPMA)-b-P(LLA) copolymers and their fluorescently labeled derivatives by facile replacement of the pentafluorophenyl groups. The fluorescence label attached to this new type of a partially degradable amphiphilic block copolymer was used to study cellular uptake in human cervix adenocarcinoma (HeLa) cells as well as aggregation behavior by fluorescence correlation spectroscopy (FCS).
We describe a synthetic pathway to functional P(HPMA)-b-P(LLA) block copolymers. The synthesis relies on a combination of ring-opening polymerization of L-lactide, conversion into a chain transfer agent (CTA) for the RAFT polymerization of pentafluorophenyl methacrylate. A series of block copolymers was prepared that exhibited molecular weights $\overline M _{\rm n}$ ranging from 7 600 to 34 300 g . mol(-1) , with moderate PDI between 1.3 and 1.45. These reactive precursor polymers have been transformed into biocompatible P(HPMA)-b-P(LLA) copolymers and their fluorescently labeled derivatives by facile replacement of the pentafluorophenyl groups. The fluorescence label attached to this new type of a partially degradable amphiphilic block copolymer was used to study cellular uptake in human cervix adenocarcinoma (HeLa) cells as well as aggregation behavior by fluorescence correlation spectroscopy (FCS).
Barz, M., Canal, F., Koynov, K., Zentel, R., and Vicent, M.J.*, Synthesis and in vitro evaluation of defined HPMA folate conjugates: influence of aggregation on folate receptor (FR) mediated cellular uptake. Biomacromolecules, 2010. 11(9): p. 2274-82. [PubMed]
 In this article we report the synthesis and in vitro evaluation of well-defined, folate functionalized and fluorescently labeled polymers based on the clinically approved N-(2-hydroxypropyl)-methacrylamide (HPMA). The polymers were prepared applying the RAFT polymerization method as well as the reactive ester approach. The molecular weights of the polymers synthesized were around 15 and 30 kDa. The total content of conjugated folate varied from 0, 5, and 10 mol %. The cellular uptake of these polymers was investigated in the folate receptor (FR)-positive human nasopharyngeal epidermal carcinoma (KB-3-1) and FR-negative human lung epithelial carcinoma (A549) cancer cell lines. In FR-positive cells, the cellular uptake of polymers depended strongly on the folate content. The conjugates with the highest folate content led to the highest level of cell-associated fluorescence. Regarding influence of molecular weight, nonsignificant differences were observed when total cell uptake was analyzed. The cellular uptake is related to the aggregate formation of the polymer conjugates, which were studied by fluorescence correlation spectroscopy (FCS). For the conjugates, we found aggregates with a diameter ranging from 11-18 nm. Much to our surprise, we found aggregates of the same size for the 30 kDa polymer bearing 5 mol % folate and for the 15 and 30 kDa conjugates with a folate content of 10 mol %. Consequently, a different conformation in solution for the different conjugates was expected. By live cell confocal fluorescence microscopy the receptor-mediated endocytosis process was observed, as colocalization with lysosomal markers was achieved. In addition, cellular uptake was not observed in FR-negative cells (A549) and can be dramatically reduced by blocking the FR with free folic acid. Our findings clearly underline the need for a minimum amount of accessible folate units to target the FR that triggers specific cellular uptake. Furthermore, it has been demonstrated that the targeting vector itself strongly influences the aggregation behavior in solution and thus determines the interaction with cells regarding cellular uptake as well as intracellular localization.
In this article we report the synthesis and in vitro evaluation of well-defined, folate functionalized and fluorescently labeled polymers based on the clinically approved N-(2-hydroxypropyl)-methacrylamide (HPMA). The polymers were prepared applying the RAFT polymerization method as well as the reactive ester approach. The molecular weights of the polymers synthesized were around 15 and 30 kDa. The total content of conjugated folate varied from 0, 5, and 10 mol %. The cellular uptake of these polymers was investigated in the folate receptor (FR)-positive human nasopharyngeal epidermal carcinoma (KB-3-1) and FR-negative human lung epithelial carcinoma (A549) cancer cell lines. In FR-positive cells, the cellular uptake of polymers depended strongly on the folate content. The conjugates with the highest folate content led to the highest level of cell-associated fluorescence. Regarding influence of molecular weight, nonsignificant differences were observed when total cell uptake was analyzed. The cellular uptake is related to the aggregate formation of the polymer conjugates, which were studied by fluorescence correlation spectroscopy (FCS). For the conjugates, we found aggregates with a diameter ranging from 11-18 nm. Much to our surprise, we found aggregates of the same size for the 30 kDa polymer bearing 5 mol % folate and for the 15 and 30 kDa conjugates with a folate content of 10 mol %. Consequently, a different conformation in solution for the different conjugates was expected. By live cell confocal fluorescence microscopy the receptor-mediated endocytosis process was observed, as colocalization with lysosomal markers was achieved. In addition, cellular uptake was not observed in FR-negative cells (A549) and can be dramatically reduced by blocking the FR with free folic acid. Our findings clearly underline the need for a minimum amount of accessible folate units to target the FR that triggers specific cellular uptake. Furthermore, it has been demonstrated that the targeting vector itself strongly influences the aggregation behavior in solution and thus determines the interaction with cells regarding cellular uptake as well as intracellular localization.
Santamaria, B., Benito-Martin, A., Ucero, A.C., Aroeira, L.S., Reyero, A., Vicent, M.J., Orzaez, M., Celdran, A., Esteban, J., Selgas, R., Ruiz-Ortega, M., Cabrera, M.L., Egido, J., Perez-Paya, E., and Ortiz, A., A nanoconjugate Apaf-1 inhibitor protects mesothelial cells from cytokine-induced injury. PLoS One, 2009. 4(8): p. e6634. [PubMed]
 BACKGROUND: Inflammation may lead to tissue injury. We have studied the modulation of inflammatory milieu-induced tissue injury, as exemplified by the mesothelium. Peritoneal dialysis is complicated by peritonitis episodes that cause loss of mesothelium. Proinflammatory cytokines are increased in the peritoneal cavity during peritonitis episodes. However there is scarce information on the modulation of cell death by combinations of cytokines and on the therapeutic targets to prevent desmesothelization. METHODOLOGY: Human mesothelial cells were cultured from effluents of stable peritoneal dialysis patients and from omentum of non-dialysis patients. Mesothelial cell death was studied in mice with S. aureus peritonitis and in mice injected with tumor necrosis factor alpha and interferon gamma. Tumor necrosis factor alpha and interferon gamma alone do not induce apoptosis in cultured mesothelial cells. By contrast, the cytokine combination increased the rate of apoptosis 2 to 3-fold over control. Cell death was associated with the activation of caspases and a pancaspase inhibitor prevented apoptosis. Specific caspase-8 and caspase-3 inhibitors were similarly effective. Co-incubation with both cytokines also impaired mesothelial wound healing in an in vitro model. However, inhibition of caspases did not improve wound healing and even impaired the long-term recovery from injury. By contrast, a polymeric nanoconjugate Apaf-1 inhibitor protected from apoptosis and allowed wound healing and long-term recovery. The Apaf-1 inhibitor also protected mesothelial cells from inflammation-induced injury in vivo in mice. CONCLUSION: Cooperation between tumor necrosis factor alpha and interferon gamma contributes to mesothelial injury and impairs the regenerative capacity of the monolayer. Caspase inhibition attenuates mesothelial cell apoptosis but does not facilitate regeneration. A drug targeting Apaf-1 allows protection from apoptosis as well as regeneration in the course of inflammation-induced tissue injury.
BACKGROUND: Inflammation may lead to tissue injury. We have studied the modulation of inflammatory milieu-induced tissue injury, as exemplified by the mesothelium. Peritoneal dialysis is complicated by peritonitis episodes that cause loss of mesothelium. Proinflammatory cytokines are increased in the peritoneal cavity during peritonitis episodes. However there is scarce information on the modulation of cell death by combinations of cytokines and on the therapeutic targets to prevent desmesothelization. METHODOLOGY: Human mesothelial cells were cultured from effluents of stable peritoneal dialysis patients and from omentum of non-dialysis patients. Mesothelial cell death was studied in mice with S. aureus peritonitis and in mice injected with tumor necrosis factor alpha and interferon gamma. Tumor necrosis factor alpha and interferon gamma alone do not induce apoptosis in cultured mesothelial cells. By contrast, the cytokine combination increased the rate of apoptosis 2 to 3-fold over control. Cell death was associated with the activation of caspases and a pancaspase inhibitor prevented apoptosis. Specific caspase-8 and caspase-3 inhibitors were similarly effective. Co-incubation with both cytokines also impaired mesothelial wound healing in an in vitro model. However, inhibition of caspases did not improve wound healing and even impaired the long-term recovery from injury. By contrast, a polymeric nanoconjugate Apaf-1 inhibitor protected from apoptosis and allowed wound healing and long-term recovery. The Apaf-1 inhibitor also protected mesothelial cells from inflammation-induced injury in vivo in mice. CONCLUSION: Cooperation between tumor necrosis factor alpha and interferon gamma contributes to mesothelial injury and impairs the regenerative capacity of the monolayer. Caspase inhibition attenuates mesothelial cell apoptosis but does not facilitate regeneration. A drug targeting Apaf-1 allows protection from apoptosis as well as regeneration in the course of inflammation-induced tissue injury.
Mondragon, L., Orzaez, M., Sanclimens, G., Moure, A., Arminan, A., Sepulveda, P., Messeguer, A., Vicent, M.J.*, and Perez-Paya, E., Modulation of cellular apoptosis with apoptotic protease-activating factor 1 (Apaf-1) inhibitors. J Med Chem, 2008. 51(3): p. 521-9. [PubMed]
 The programmed cell death or apoptosis plays both physiological and pathological roles in biology. Anomalous activation of apoptosis has been associated with malignancies. The intrinsic mitochondrial pathway of apoptosis activation occurs through a multiprotein complex named the apoptosome. We have discovered molecules that bind to a central protein component of the apoptosome, Apaf-1, and inhibits its activity. These new first-in-class apoptosome inhibitors have been further improved by modifications directed to enhance their cellular penetration to yield compounds that decrease cell death, both in cellular models of apoptosis and in neonatal rat cardiomyocytes under hypoxic conditions.
The programmed cell death or apoptosis plays both physiological and pathological roles in biology. Anomalous activation of apoptosis has been associated with malignancies. The intrinsic mitochondrial pathway of apoptosis activation occurs through a multiprotein complex named the apoptosome. We have discovered molecules that bind to a central protein component of the apoptosome, Apaf-1, and inhibits its activity. These new first-in-class apoptosome inhibitors have been further improved by modifications directed to enhance their cellular penetration to yield compounds that decrease cell death, both in cellular models of apoptosis and in neonatal rat cardiomyocytes under hypoxic conditions.
Duncan, R., Gilbert, H.R., Carbajo, R.J., and Vicent, M.J.*, Polymer masked-unmasked protein therapy. 1. Bioresponsive dextrin-trypsin and -melanocyte stimulating hormone conjugates designed for alpha-amylase activation. Biomacromolecules, 2008. 9(4): p. 1146-54. [PubMed]
 Polymer-protein conjugation, particularly PEGylation, is well-established as a means of increasing circulation time, reducing antigenicity, and improving the stability of protein therapeutics. However, PEG has limitations including lack of polymer biodegradability, and conjugation can diminish or modify protein activity. The aim of this study was to explore a novel approach for polymer-protein modification called polymer-masking-unmasking-protein therapy (PUMPT), the hypothesis being that conjugation of a biodegradable polymer to a protein would protect it and mask activity in transit, while enabling controlled reinstatement of activity at the target site by triggered degradation of the polymeric component. To test this hypothesis, dextrin (alpha-1,4 polyglucose, a natural polymer degraded by alpha-amylase) was conjugated to trypsin as a model enzyme or to melanocyte stimulating hormone (MSH) as a model receptor-binding ligand. The effect of dextrin molecular weight (7700, and 47200 g/mol) and degree of succinoylation (9-32 mol %) on its ability to mask/unmask trypsin activity was assessed using N-benzoyl-L-arginine-p-nitroanilide (L-BAPNA). Dextrin conjugation reduced enzyme activity by 34-69% depending on the molecular weight and degree of succinoylation of dextrin. However, incubation with alpha-amylase led to reinstatement of activity to a maximum of 92-115%. The highest molecular dextrin (26 mol % succinoylation) gave optimum trypsin masking-unmasking. This intermediate was used to synthesize a dextrin-MSH conjugate (dextrin Mw = 47200 g/mol; MSH content 37 wt %), and its biological activity (+/-alpha-amylase) was assessed by measuring melanin production by murine melanoma (B16F10) cells. Conjugation reduced melanin production to 11%, but addition of alpha-amylase was able to restore activity to 33% of the control value. These were the first studies to confirm the potential of PUMPT for further application to clinically important protein therapeutics. The choice of masking polymer, activation mechanism, and the rate of unmasking can be tailored to therapeutic application.
Polymer-protein conjugation, particularly PEGylation, is well-established as a means of increasing circulation time, reducing antigenicity, and improving the stability of protein therapeutics. However, PEG has limitations including lack of polymer biodegradability, and conjugation can diminish or modify protein activity. The aim of this study was to explore a novel approach for polymer-protein modification called polymer-masking-unmasking-protein therapy (PUMPT), the hypothesis being that conjugation of a biodegradable polymer to a protein would protect it and mask activity in transit, while enabling controlled reinstatement of activity at the target site by triggered degradation of the polymeric component. To test this hypothesis, dextrin (alpha-1,4 polyglucose, a natural polymer degraded by alpha-amylase) was conjugated to trypsin as a model enzyme or to melanocyte stimulating hormone (MSH) as a model receptor-binding ligand. The effect of dextrin molecular weight (7700, and 47200 g/mol) and degree of succinoylation (9-32 mol %) on its ability to mask/unmask trypsin activity was assessed using N-benzoyl-L-arginine-p-nitroanilide (L-BAPNA). Dextrin conjugation reduced enzyme activity by 34-69% depending on the molecular weight and degree of succinoylation of dextrin. However, incubation with alpha-amylase led to reinstatement of activity to a maximum of 92-115%. The highest molecular dextrin (26 mol % succinoylation) gave optimum trypsin masking-unmasking. This intermediate was used to synthesize a dextrin-MSH conjugate (dextrin Mw = 47200 g/mol; MSH content 37 wt %), and its biological activity (+/-alpha-amylase) was assessed by measuring melanin production by murine melanoma (B16F10) cells. Conjugation reduced melanin production to 11%, but addition of alpha-amylase was able to restore activity to 33% of the control value. These were the first studies to confirm the potential of PUMPT for further application to clinically important protein therapeutics. The choice of masking polymer, activation mechanism, and the rate of unmasking can be tailored to therapeutic application.
Paul, A., Vicent, M.J.*, and Duncan, R., Using small-angle neutron scattering to study the solution conformation of N-(2-hydroxypropyl)methacrylamide copolymer-doxorubicin conjugates. Biomacromolecules, 2007. 8(5): p. 1573-9. [PubMed]
 Our past research developed two N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin (Dox) conjugates that became the first synthetic polymer-anticancer conjugates to be evaluated clinically. The first, FCE28068, contained Dox bound to the polymeric carrier via a tetrapeptidic linker (glycine-phenylalanine-leucine-glycine (GFLG)) (Mw approximately 30,000 g/mol; approximately 8 wt % drug), and the second, FCE28069, contained additionally galactosamine (Gal) (Mw approximately 30,000 g/mol; approximately 7.5 wt % Dox) again bound by a GFLG linker. Galactosamine was included to promote hepatocyte/hepatoma targeting via the asialoglycoprotein receptor. Both conjugates showed antitumor activity and were clinically less toxic than free Dox (2-5 fold). However, despite their similar chemical characteristics, the conjugates displayed a significantly different maximum-tolerated dose (MTD) in patients. The aim of this study, therefore, was to use small-angle neutron scattering (SANS) to explore the solution behavior of a small library of HPMA polymer conjugates including FCE28068, FCE28069, and their pharmaceutical formulations, plus as reference compounds HPMA copolymer-GFLG conjugates containing aminopropanol (Ap) or galactosamine (Gal) alone (i.e., without Dox). The SANS data obtained showed that HPMA copolymer-GFLG-Ap conjugates (containing 5 and 10 mol % side chains) showed evidence of polymer aggregation, however, no indication of aggregation was observed for FCE28068 and FCE28069 over the concentration range studied (2.5-50 mg/mL). Clear differences in the scattering behavior for the two conjugates were observed at equivalent concentration. Data were best fitted by a model for polydisperse Gaussian coils, and the HPMA copolymer-Dox conjugate with Gal (FCE28069) exhibited a larger radius of gyration (Rg) (by approximately 2.5 nm) compared to FCE28068. In conclusion, we have shown that SANS will be a valuable tool to elucidate conformation-performance relationships for polymer-drug conjugates.
Our past research developed two N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin (Dox) conjugates that became the first synthetic polymer-anticancer conjugates to be evaluated clinically. The first, FCE28068, contained Dox bound to the polymeric carrier via a tetrapeptidic linker (glycine-phenylalanine-leucine-glycine (GFLG)) (Mw approximately 30,000 g/mol; approximately 8 wt % drug), and the second, FCE28069, contained additionally galactosamine (Gal) (Mw approximately 30,000 g/mol; approximately 7.5 wt % Dox) again bound by a GFLG linker. Galactosamine was included to promote hepatocyte/hepatoma targeting via the asialoglycoprotein receptor. Both conjugates showed antitumor activity and were clinically less toxic than free Dox (2-5 fold). However, despite their similar chemical characteristics, the conjugates displayed a significantly different maximum-tolerated dose (MTD) in patients. The aim of this study, therefore, was to use small-angle neutron scattering (SANS) to explore the solution behavior of a small library of HPMA polymer conjugates including FCE28068, FCE28069, and their pharmaceutical formulations, plus as reference compounds HPMA copolymer-GFLG conjugates containing aminopropanol (Ap) or galactosamine (Gal) alone (i.e., without Dox). The SANS data obtained showed that HPMA copolymer-GFLG-Ap conjugates (containing 5 and 10 mol % side chains) showed evidence of polymer aggregation, however, no indication of aggregation was observed for FCE28068 and FCE28069 over the concentration range studied (2.5-50 mg/mL). Clear differences in the scattering behavior for the two conjugates were observed at equivalent concentration. Data were best fitted by a model for polydisperse Gaussian coils, and the HPMA copolymer-Dox conjugate with Gal (FCE28069) exhibited a larger radius of gyration (Rg) (by approximately 2.5 nm) compared to FCE28068. In conclusion, we have shown that SANS will be a valuable tool to elucidate conformation-performance relationships for polymer-drug conjugates.
Orzaez, M., Mondragon, L., Marzo, I., Sanclimens, G., Messeguer, A., Perez-Paya, E., and Vicent, M.J.*, Conjugation of a novel Apaf-1 inhibitor to peptide-based cell-membrane transporters: effective methods to improve inhibition of mitochondria-mediated apoptosis. Peptides, 2007. 28(5): p. 958-68. [PubMed]
We have identified a family of peptoids that inhibits in vitro the activity of the apoptosome, a macromolecular complex that activates mitochondrial-dependent apoptosis pathways. The analysis of peptide-based cell compatible delivery systems of the most active peptoid is presented. The active peptoid was then fused to cell penetrating peptides (CPP) as penetratin (PEN-peptoid) and HIV-1 TAT (TAT-peptoid). PEN-peptoid showed greater cell viability and as a consequence better efficiency as an apoptosis inhibitor than the TAT-peptoid. The intracellular trafficking of both inhibitors was studied by flow cytometry and confocal fluorescence microscopy. Finally, the influence of the cargo (peptoid) molecules on the conformational behavior of the CPP in buffers and in membrane mimetic environments was analyzed using circular dichroism (CD) spectroscopy.
Greco, F., Vicent, M.J., Gee, S., Jones, A.T., Gee, J., Nicholson, R.I., and Duncan, R., Investigating the mechanism of enhanced cytotoxicity of HPMA copolymer-Dox-AGM in breast cancer cells. J Control Release, 2007. 117(1): p. 28-39. [PubMed]
Recently we have described an HPMA copolymer conjugate carrying both the aromatase inhibitor aminoglutethimide (AGM) and doxorubicin (Dox) as combination therapy. This showed markedly enhanced in vitro cytotoxicity compared to the HPMA copolymer-Dox (FCE28068), a conjugate that demonstrated activity in chemotherapy refractory breast cancer patients during early clinical trials. To better understand the superior activity of HPMA copolymer-Dox-AGM, here experiments were undertaken using MCF-7 and MCF-7ca (aromatase-transfected) breast cancer cell lines to: further probe the synergistic cytotoxic effects of AGM and Dox in free and conjugated form; to compare the endocytic properties of HPMA copolymer-Dox-AGM and HPMA copolymer-Dox (binding, rate and mechanism of cellular uptake); the rate of drug liberation by lysosomal thiol-dependant proteases (i.e. conjugate activation), and also, using immunocytochemistry, to compare their molecular mechanism of action. It was clearly shown that attachment of both drugs to the same polymer backbone was a requirement for enhanced cytotoxicity. FACS studies indicated both conjugates have a similar pattern of cell binding and endocytic uptake (at least partially via a cholesterol-dependent pathway), however, the pattern of enzyme-mediated drug liberation was distinctly different. Dox release from PK1 was linear with time, whereas the release of both Dox and AGM from HPMA copolymer-Dox-AGM was not, and the initial rate of AGM release was much faster than that seen for the anthracycline. Immunocytochemistry showed that both conjugates decreased the expression of ki67. However, this effect was more marked for HPMA copolymer-Dox-AGM and, moreover, only this conjugate decreased the expression of the anti-apoptotic protein bcl-2. In conclusion, the superior in vitro activity of HPMA copolymer-Dox-AGM cannot be attributed to differences in endocytic uptake, and it seems likely that the synergistic effect of Dox and AGM is due to the kinetics of intracellular drug liberation which leads to enhanced activity.
Vicent, M.J.* and Perez-Paya, E., Poly-L-glutamic acid (PGA) aided inhibitors of apoptotic protease activating factor 1 (Apaf-1): an antiapoptotic polymeric nanomedicine. J Med Chem, 2006. 49(13): p. 3763-5. [PubMed]
 An antiapoptotic polymeric nanomedicine, PGA-peptoid 1, has been developed by the conjugation of a novel apoptotic protease activating factor 1 (Apaf-1) inhibitor (peptoid 1) to poly-L-glutamic acid (PGA). Full structural and biophysical characterization of the conjugate by different techniques has been accomplished. This macromolecule clearly enhances the antiapoptotic activity of peptoid 1 in different cell models.
An antiapoptotic polymeric nanomedicine, PGA-peptoid 1, has been developed by the conjugation of a novel apoptotic protease activating factor 1 (Apaf-1) inhibitor (peptoid 1) to poly-L-glutamic acid (PGA). Full structural and biophysical characterization of the conjugate by different techniques has been accomplished. This macromolecule clearly enhances the antiapoptotic activity of peptoid 1 in different cell models.
Malet, G., Martin, A.G., Orzaez, M., Vicent, M.J., Masip, I., Sanclimens, G., Ferrer-Montiel, A., Mingarro, I., Messeguer, A., Fearnhead, H.O., and Perez-Paya, E., Small molecule inhibitors of Apaf-1-related caspase- 3/-9 activation that control mitochondrial-dependent apoptosis. Cell Death Differ, 2006. 13(9): p. 1523-32. [PubMed]
 Apoptosis is a biological process relevant to human disease states that is strongly regulated through protein-protein complex formation. These complexes represent interesting points of chemical intervention for the development of molecules that could modulate cellular apoptosis. The apoptosome is a holoenzyme multiprotein complex formed by cytochrome c-activated Apaf-1 (apoptotic protease-activating factor), dATP and procaspase-9 that link mitochondria disfunction with activation of the effector caspases and in turn is of interest for the development of apoptotic modulators. In the present study we describe the identification of compounds that inhibit the apoptosome-mediated activation of procaspase-9 from the screening of a diversity-oriented chemical library. The active compounds rescued from the library were chemically optimised to obtain molecules that bind to both recombinant and human endogenous Apaf-1 in a cytochrome c-noncompetitive mechanism that inhibits the recruitment of procaspase-9 by the apoptosome. These newly identified Apaf-1 ligands decrease the apoptotic phenotype in mitochondrial-mediated models of cellular apoptosis.
Apoptosis is a biological process relevant to human disease states that is strongly regulated through protein-protein complex formation. These complexes represent interesting points of chemical intervention for the development of molecules that could modulate cellular apoptosis. The apoptosome is a holoenzyme multiprotein complex formed by cytochrome c-activated Apaf-1 (apoptotic protease-activating factor), dATP and procaspase-9 that link mitochondria disfunction with activation of the effector caspases and in turn is of interest for the development of apoptotic modulators. In the present study we describe the identification of compounds that inhibit the apoptosome-mediated activation of procaspase-9 from the screening of a diversity-oriented chemical library. The active compounds rescued from the library were chemically optimised to obtain molecules that bind to both recombinant and human endogenous Apaf-1 in a cytochrome c-noncompetitive mechanism that inhibits the recruitment of procaspase-9 by the apoptosome. These newly identified Apaf-1 ligands decrease the apoptotic phenotype in mitochondrial-mediated models of cellular apoptosis.
Greco, F., Vicent, M.J., Penning, N.A., Nicholson, R.I., and Duncan, R., HPMA copolymer-aminoglutethimide conjugates inhibit aromatase in MCF-7 cell lines. J Drug Target, 2005. 13(8-9): p. 459-70. [PubMed]
N-(2-Hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin (Dox) has already shown clinical activity in breast cancer patients. Moreover, we have recently found that an HPMA conjugate containing a combination of both Dox and the aromatase inhibitor aminoglutethimide (AGM) shows significantly increased anti-tumour activity in vitro. To better understand the mechanism of action of HPMA copolymer-AGM conjugates several models were used here to investigate their effect on cell growth and aromatase inhibition. Cytotoxicity of HPMA copolymer conjugates containing AGM, Dox and also the combination AGM-Dox was determined by MTT assay in MCF-7 and MCF-7ca cells. Androstenedione (5 x 10(- 8) M) stimulates the growth of MCF-7ca cells. Both free AGM and polymer-bound AGM (0.2-0.4 mg/ml) were shown to block this mitogenic activity. When MCF-7ca cells were incubated [(3)H]androstenedione both AGM and HPMA copolymer-GFLG-AGM (0.2 mg/ml AGM-equiv.) showed the ability to inhibit aromatase. Although, free AGM was able to inhibit isolated human placental microsomal aromatase in a concentration dependent manner, polymer-bound AGM was not, suggesting that drug release is essential for activity of the conjugate. HPMA copolymer conjugates containing aromatase inhibitors have potential for the treatment of hormone-dependant cancers, and it would be particularly interesting to explore further as potential therapies in post-menopausal women as components of combination therapy.
Vicent, M.J.*, Greco, F., Nicholson, R.I., Paul, A., Griffiths, P.C., and Duncan, R., Polymer therapeutics designed for a combination therapy of hormone-dependent cancer. Angew Chem Int Ed Engl, 2005. 44(26): p. 4061-6. [PubMed]
 Designer drug: A polymer therapeutic was designed for a combination therapy of breast cancer. N-(2-Hydroxypropyl)methacrylamide was used as the model polymer platform to prepare a unimolecular polymer conjugate (see picture, radius of gyration: 12.8 nm) that combines an endocrine (the aromatase inhibitor aminoglutethimide, blue) and a chemotherapeutic agent (the anthraxcycline antibiotic doxorubicin, red).
Designer drug: A polymer therapeutic was designed for a combination therapy of breast cancer. N-(2-Hydroxypropyl)methacrylamide was used as the model polymer platform to prepare a unimolecular polymer conjugate (see picture, radius of gyration: 12.8 nm) that combines an endocrine (the aromatase inhibitor aminoglutethimide, blue) and a chemotherapeutic agent (the anthraxcycline antibiotic doxorubicin, red).
Vicent, M.J.*, Tomlinson, R., Brocchini, S., and Duncan, R., Polyacetal-diethylstilboestrol: a polymeric drug designed for pH-triggered activation. J Drug Target, 2004. 12(8): p. 491-501. [PubMed]
Those polymer anticancer-drug conjugates currently undergoing clinical evaluation have a tripartite structure; a water-soluble polymer, an anticancer agent and a pendant linker. To simplify the construct it would be attractive to develop anticancer polymer therapeutics that contain the bioactive agent as an integral part of the polymer backbone. The aim of this study was to utilise the reaction between a divinyl ethers and diols, to synthesise polyacetals incorporating a drug with bis-hydroxyl functionality into the polymer backbone. Degradation of the polymer backbone in the acidic environment of the lysosome or the extracellular fluid of some tumours would then trigger drug release eliminating the need for a biodegradable linker. A tert-polymerisation approach was used to incorporate non-steroidal oestrogen diethylstilboestrol (DES) into the mainchain of water-soluble polyacetals synthesised using as co-monomer PEG of Mw 2900 or 3400 g/mol. When PEG2900 was used the resultant polymer had a Mw of 18,900 g/mol, a Mw/Mn of 1.9 and a DES loading 4.3 wt.%. With PEG3400 the polymer Mw was 43,000 g/mol, Mw/Mn=1.8 and it had a DES loading 4.7 wt.%. 1H-NMR confirmed the presence of two distinct sets of acetal peaks, which correspond to the two possible mainchain acetals; from PEG at 1.25-1.3(d) and 4.7-4.8(q) ppm and from DES at 1.55-1.6(d) and 5.4-5.5(q) ppm. These were consistent with the acetal signals observed for the non-water-soluble co-polymer DES: tri(ethylene glycol) divinyl ether (TEGDVE) (1 : 2, Mw=6859 g/mol, Mw/Mn=1.3). When evaluated in vitro, the DES-polyacetal displayed greater cytotoxicity than DES against human and murine tumour cell lines (IC50=48 and 420 microg/ml against MCF-7 human breast cancer cells and IC50=97 and 560 microg/ml against B16F10 murine melanoma cells, respectively). These polymers showed no significant haemolysis at concentrations up to 20 mg/ml confirming suitability for further in vivo evaluation. An enhanced rate of hydrolytic degradation of the polymer backbone was seen at pH 5.5, (65% trans-DES released in 96 h), compared to pH 7.4 (4% trans-DES released in 96 h). These bioresponsive DES-polyacetals tert-polymers are the first water-soluble anticancer polymeric drugs designed for acidic pH-triggered release of a drug incorporated into the polymer mainchain. Their in vitro characteristics suggest further in vivo evaluation is warranted.
Vicent, M.J.*, Manzanaro, S., de la Fuente, J.A., and Duncan, R., HPMA copolymer-1,5-diazaanthraquinone conjugates as novel anticancer therapeutics. J Drug Target, 2004. 12(8): p. 503-15. [PubMed]
1,5-diazaanthraquinones (DAQs) are promising anticancer drugs, however, their clinical potential is limited due to poor solubility. Conjugation of anticancer agents to hydrophilic water-soluble polymers can overcome this problem and has already been used to generate conjugates with demonstrated clinical benefit. Here a library of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer conjugates containing a novel amino-functionalised 1,5-diazaanthraquinone derivative (amino-DAQ) have been synthesised. The conjugates were fully characterised by UV, HPLC, SEC, FT-Raman and NMR spectroscopy. Conjugation to HPMA copolymers improved amino-DAQ aqueous solubility (>7-fold). The HPMA copolymer-amino-DAQ conjugates were slightly less haemolytic than the parent compound (2% Hb released in 1 h for conjugate HPMA copolymer-GFLG (5 mol%)-amino-DAQ conjugate compared to 13% obtained with amino-DAQ). When conjugates were incubated with isolated rat liver lysosomal enzymes (Tritosomes) the rate of amino-DAQ release was influenced by both drug loading and the composition of the peptidyl side chain used to link the drug to the carrier. The higher the drug loading the lower the rate of drug release. Whereas the GG linker did not release amino-DAQ, up to 26% of the amino-DAQ was released from a GFLG linker over 24 h. The in vitro cytotoxicity of these conjugates was evaluated against two different cell lines, B16F10 murine melanoma and MCF-7 human breast cancer cells. HPMA copolymer-amino-DAQ conjugates, which are internalised by cells by the endocytic pathway, showed much lower in vitro cytotoxicity (IC50 for HPMA copolymer-GFLG (5 mol%)-amino-DAQ conjugate>397 microM drug-equiv.) than the free drug (the IC50 for amino-DAQ was 12.6 and 2.8 microM against the B16F10 murine melanoma and the MCF-7 breast cancer cell line, respectively). Nonetheless, the observed lysosomal activation of the HPMA copolymer-GFLG-amino-DAQ conjugates, suggests that evaluation of the antitumour potential in vivo is warranted.
Proceedings
Polypeptide-based Conjugates as Versatile Therapeutics
María J. Vicent
ICONAN2019 October 16-18, Munich (Germany)
[PDF]
Targeting Tumor Microenvironment with Rationally Designed Polypeptide-based Conjugates
María J. Vicent
NanoBio&Med 2018 November 20 – 22, Barcelona (Spain)
[PDF]
PO-416 - A novel multifunctional polypeptide-based platform as an immunotherapeutic approach for melanoma
L Moura, A Duro-Castano, C Peres, E Gallon, A Matos, MJ Vicent, H Florindo
ESMO Open 2018;3:doi: 10.1136/esmoopen-2018-EACR25.927
Introduction Melanoma is the most dangerous type of skin cancer and novel treatments are needed. Alternative therapeutics should be devised isolated or in combination with targeted immunotherapies, to efficiently stimulate specific anti-tumour responses. Branched polypeptides exhibit advanced engineered complexity and unique structural properties inaccessible to linear polymers that make them ideal drug delivery systems with enhanced biological performance. Branched nanosystems have the ability to activate immune cells, as dendritic cells (DC) and natural killer (NK) cells, constituting potential platforms to modulate the release profile of loaded molecules, including tumour associated antigens (TAA), adjuvants and drugs. This work aims to evaluate the in vivo anti-tumour efficacy of peptide-1 -conjugated polypeptide (pept-1-BP), with special emphasis on their impact on the modulation of the immune cell function.
Material and methods BP were synthesised and conjugated with the peptide-1 (pept-1-BP) via reductive-sensitive disulfide linker. To address in vitro and in vivo studies, Cy5.5 was conjugated to platform. To evaluate the effect of the conjugate on melanoma tumour growth, B16.F10 cells were implanted subcutaneously into C57BL/6 mice. At day 7, animals were injected with two doses (1 week apart) of 100 µL of PBS, Toll-like receptor ligands CpG (20 µg/dose) and Poly I:C (40 µg/dose) in solution, BP backbone (575 µg/dose) and pept-1-BP (575 µg/dose) mixed with adjuvants. Every 2 days, weight of the mice and tumour growth was followed. At day 21, mice were sacrificed and tumour and lymph nodes were collected. A cell suspension from tumour cells and lymph nodes of each animal was prepared and analysed for infiltrated lymphocytes (CD45.1, CD3e, CD8α, CD4, CD107, PD-1, CTLA-4) by flow cytometry.
Results and discussions The BP presented a size of 81.86±1.63 nm and a zeta potential of −45.10±1.72 mV, while pept-1-BP showed a mean average diameter of 104.1±2.21 nm and a zeta potential of −24.8±0.64 mV, with a pept-1 loading efficiency of 8.7% (w/w).
In vivo results showed a significant reduction of tumour size in conjugate treated mice compared with the other groups. In addition, the FACS analysis of infiltrating lymphocytes within tumour site evidenced an increased expression for CD4, CD8α and NK cells.
Conclusion Overall, our results support the promising use of this novel conjugate for the delivery of TAA, as an effective anti-tumour immune therapeutic strategy able to decrease and control of tumour growth.
Abstract 3726: Design Of Personalised Polymer Based Combination Therapeutics for Advanced Stage Breast Cancer Patients
Ana Armiñán, Zoraida Andreu, Juan J Arroyo Crespo, David Charbonnier, Esther Masiá, Fernanda Rodriguez-Otomin, Aroa Duro-Castano, Vicent J Nebot and Maria J Vicent
DOI: 10.1158/1538-7445.AM2018-3726 Published July 2018
Proceedings: AACR Annual Meeting 2018; April 14-18, 2018; Chicago, IL
Abstract
Our objective is to engineer tumour-targeted polymer-based combination therapies specifically designed to treat metastatic breast cancer (BC) in a personalised manner. Our strategy is to develop novel multicomponent polymer conjugates and assess structure activity relationships in clinically relevant models to understand mechanisms of action. Furthermore, we are searching for novel drug combinations, including tumour-derived exosome release pathway inhibitors due to their association with metastasis and tumour drug resistance mechanisms. NCA polymerization techniques have allowed us to precisely control the synthesis of well-defined star-based (STP) and linear (LTP) polypeptidic architectures. After fluorescence labelling, we studied our systems in vitro demonstrating a much more rapid uptake for STP through clathrin-coated mediated endocytosis. pK and biodistribution in healthy mice revealed renal excretion profiles and greater terminal and accumulation half-lives for STP architectures when compared to the LTP. Remarkably, we also observed clear accumulation in immune system-related organs including the spleen and lymph nodes (LN) (up to 40% ID/g tissue accumulation in the LN after i.v. administration). This is possibly due to their inherent structural and morphological features, such as their size (≈100nm), highly negative z-potential values, and/or hydrophilic surfaces. These data highlight the great potential of our stabilized self-assemblies as carriers to target LN metastasis, cancer immunotherapy, or immune system-related approaches, such as vaccination. In parallel, we have performed a High Throughput Screening to select synergistic drug combinations to be used in polymer-based combination approaches through rationally designed linkers that confer adequate drug release kinetics. To perform this approach we selected four metastatic human BC cell lines representing clinical BC subtypes. All cell models have been fully characterized regarding their Cathepsin B activity, intracellular pH, as well as oestrogen, progesterone, Her2 receptors, GSH and exosomes levels; all representing patient-specific biomarkers. Cell viability and exosomes release modulation have been studied following treatments and several drug combinations have been selected for each specific BC subtype. With selected drug combinations different linking chemistry has been designed. We have studied a combination conjugate with the chemotherapeutic agent conjugated to PGA through two different length pH-labile hydrazone linkers. This provided different therapeutic outputs in cells and in a metastatic immunocompetent orthotopic breast cancer model, not only for the primary tumor but also for metastasis progression. The results obtained so far open up a wide range of opportunities for the currently unsuccessful clinical approaches to target LN metastasis and cancer immunotherapy.
MP070 – A Polymer Conjugate Nanomedicine Inhibits LPS-Induced MAPK Activation and Reduces Endotoxemia-Mediated Kidney Inflammation.
Cristian González-Guerrero, María Jesús Vicent, Alberto Ortiz, Adrian Ramos
Nephrology Dialysis Transplantation, Volume 32, Issue suppl_3, 1 May 2017, Pages iii451, https://doi.org/10.1093/ndt/gfx162.MP070
INTRODUCTION AND AIMS: Endotoxemia-associated acute kidney injury during sepsis is a major cause of mortality in ICU patients by contributing to multiple organ failure. General and kidney deregulated immune responses provoked by endotoxemia involves a widespread activation of the innate immune system. Toll-like receptor 4 (TLR4) is a key mediator of innate immunity and amplifies inflammation in AKI. Moreover, kidney TLR4 expression is increased in experimental endotoxemia and TLR4 targeting is protective. However, no drugs targeting TLR4 are in clinical use. We previously reported that the experimental nanomedicine QM56 inhibits NF-κB-dependent proinflammatory responses and protects from experimental nephrotoxic AKI (Ucero A. et al., Plos One 2013;8(1):e51992). We have now explored whether QM56 interferes with LPS-induced TLR4 signaling and protects from endotoxemia-induced renal inflammation and injury.
METHODS: Cell culture assays were done in the mouse cell lines of kidney tubules (MCT), endothelium (MS1) and macrophages (RAW264.7) and also in primary cultures of thyoglicollate-elicited peritoneal macrophages. Cells were treated with LPS (0.1 – 1µg/ml) or cotreated with QM56 (20 µM) and LPS (1µg/ml) and further analyzed for both TLR4-dependent proinflammatory pathway regulation and NF-κB-dependent cytokine expression. Results from cell culture assays were validated in groups of C57BL/6 mice treated with LPS (0.4 mg/kg, i.p.) alone or cotreated with LPS and QM56 (2.5 mg/kg, r.o., injected 5 hours before and also along with LPS). The control group was treated with drug vehicle. After treatment, mice were euthanized under general anesthesia and kidneys analyzed for gene (quantitative RT-PCR) and protein (immunohistochemistry) expression.
RESULTS: In cultured murine tubular and endothelial cells, QM56 inhibited the LPS-induced gene expression of proinflammatory cytokines (MCP-1, CXCL10, IL-6 and TNF-α) and biomarkers of endothelial activation (ICAM-1, VCAM-1, E-selectin). Similar findings were obtained in RAW264.7 macrophage cells and in peritoneal macrophages. In RAW264.7 cells, QM56 also inhibited TLR4- and TRIF-dependent interferon pathway gene expression (IRF1, IRF7, Ifit1). QM56 did not inhibit the LPS-induced IκBα phosphorylation and p65 nuclear translocation but instead decreased the phosphorylation/activation of the MAPKs, JNK, ERK and p38. In C57BL/6 mice injected with LPS (0.4 mg/kg, i.p.), QM56 prevented the LPS-induced progressive neutrophil and macrophage kidney infiltration after 4 and 16 h treatment and preserved renal function as assessed by serum urea. QM56 also decreased kidney MCP-1 and VCAM-1 gene expression as well as the expression of early tubular injury marker KIM-1.
CONCLUSIONS: Overall, this study identifies QM56 as a potential therapy for endotoxemia and sepsis, which prevents kidney injury by inhibiting MAP kinases and by downregulating the expression NF-κB-dependent proinflammatory genes.
Polymer therapeutics and stem cell therapies as a combinatorial approach for the treatment of chronic spinal cord injuries.
Nebot, V., R. Requejo-Aguilar, A. Arminan, O. Zagorodko, A. Alastrue-Agudo, V. Moreno-Manzano and M. Vicent (2017).
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY, AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA.
Versatile star-shaped polypeptide conjugates with controlled self-assembly as therapeutics.
Vicent, M. (2017).
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY, AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA.
Covalently captured dynamic self-assembled star-shaped polyglutamates as drug delivery vehicles.
Duro-Castano, A., V. Nebot, A. Nino-Pariente, N. Feiner, J. Arroyo, A. Paul, A. Arminan, L. Albertazzi and M. Vicent (2017).
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY, AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA.
PA-Curcumin Conjugate and Combined Therapy with Ependymal/Progenitor Cells Enhance Recovery from Spinal Cord Injury.
Requejo, R., A. Alastrue, M. Cases, M. Vicent, R. England and V. Moreno (2016).
TISSUE ENGINEERING PART A, MARY ANN LIEBERT, INC 140 HUGUENOT STREET, 3RD FL, NEW ROCHELLE, NY 10801 USA.
Abstract LB-196: Preventing breast cancer metastases with an anti-angiogenic and anticancer RGD-bearing nanomedicine
Anat Eldar-Boock, Dikla Ben-Shushan, Joaquin Sanchis, Ruth Lupu, Maria J. Vicent and Ronit Satchi-Fainaro
DOI: 10.1158/1538-7445.AM2014-LB-196 Published October 2014
Abstract
Overcoming drug resistance, emerging either on recurring cancer or metastasis, is a major challenge of cancer treatments. The combination of anti-angiogenic agents with chemotherapeutic ones offers a promising therapeutic approach. Such combination can be achieved exploiting the multivalency of polymer therapeutics. We have recently showed that polymer conjugation of paclitaxel (PTX), a potent cytotoxic and anti-angiogenic drug, improved its pharmacokinetic profile. We hypothesized that actively targeting PTX to αvβ3-integrin, presents an attractive therapeutic strategy for breast cancer. Interestingly, overexpression of αvβ3-integrin, occurring on tumor endothelial and some epithelial cells during tumor growth, invasion, and metastasis, was found to correlate with PTX-resistance of breast cancer cells.
We designed and synthesized a novel polyglutamic acid (PGA)-PTX-E-[c(RGDfK)2] nano-sized conjugate. Polymer conjugation converted PTX to a water-soluble macromolecule, which passively targeted the tumor tissue exploiting the enhanced permeability and retention (EPR) effect. The E-[c(RGDfK)2] was utilized as an additional active targeting moiety to αvβ3 integrin. PGA is enzymatically-degradable by cathepsin B, leading to PTX release. PGA-PTX-E-[c(RGDfK)2] displayed a potent anti-angiogenic activity, determined by several well-established in vitro assays. Preferential tumor accumulation of the RGD-bearing conjugate in orthotopic mammary tumors inoculated in mice, lead to enhanced antitumor efficacy and a marked decrease in toxicity compared with free PTX. We found that metastasis establishment was dependent on αvβ3-integrin expression as determined in vitro and ex vivo. PGA-PTX-E-[c(RGDfK)2] conjugate inhibited metastatic growth in an experimental breast cancer metastases model in mice.
Taken together, inclusion of an active targeting moiety to integrin expressing-cells has the potential to decrease breast cancer metastases establishment by displaying an anti-angiogenic and anticancer activity.
Abstract 5225: Correlation between αvβ3 integrin expression, paclitaxel resistance and RGD-bearing conjugate efficacy
Anat Eldar-Boock, Joaquin Sanchis, Ruth Lupu, Maria J. Vicent and Ronit Satchi-Fainaro
DOI: 10.1158/1538-7445.AM2012-5225 Published April 2012
Abstract
Overcoming drug resistance, emerging either on recurring cancer or metastasis, is a major target of cancer treatments. The combination of anti-angiogenic therapy with cytotoxic therapy offers a promising therapeutic approach. Paclitaxel (PTX) is a widely-used potent cytotoxic drug, which also exhibits anti-angiogenic activity at low doses. The use of the hydrophobic PTX to its full potential is limited by severe side effects, caused by the drug and its solubilizing agents. Integrins play a key role in cell matrix interactions. The highly restricted expression of integrin αvβ3, overexpressed on tumor endothelial and some epithelial cells, during tumor growth, invasion, and metastasis present an interesting molecular target for metastatic breast tumors. In addition, PTX acquired resistance correlated with integrin αvβ3 overexpression on breast cancer cells. We designed and synthesized a novel polyglutamic acid (PGA)-PTX-E-[c(RGDfK)2] nano-scaled conjugate. Polymer conjugation converted PTX to a water-soluble macromolecule, which passively targeted the tumor tissue exploiting the enhanced permeability and retention (EPR) effect, while extravasating via the leaky tumor neovasculature. The E-[c(RGDfK)2] enhanced the effects previously seen for PGA-PTX alone, utilizing the additional active targeting to the αvβ3 integrin. PGA is enzymatically-degradable by cathepsin B, leading to PTX release. PGA-PTX-E-[c(RGDfK)2] displayed a potent anti-angiogenic therapy, determined by several, well-established, in vitro assays. Mice bearing orthotopic mammary tumors demonstrated preferential tumor accumulation of the RGD-bearing conjugate, leading to enhanced antitumor efficacy and a marked decreased in toxicity compared with free PTX. The correlation between αvβ3 integrin expression on tumor cells and acquired PTX-resistance was determined by in vitro manipulation of αvβ3 overexpressing-cells and by examining subsets of primary versus metastatic or recurring tumors of PTX-treated patients as well as free and conjugated-PTX-treated tumor-bearing mice. Taken together, our conjugate alters the pharmacokinetics of free PTX. Inclusion of an active targeting moiety to integrin expressing-cells, have the potential to manipulate and overcome acquired drug resistance, which will hopefully warrant it as a novel targeted, anti-angiogenic and anticancer therapy.
94 EVALUATION OF APOPTOSIS INHIBITION USING APAF-1 INHIBITORS (PEPTOID 1) IN AN ISCHAEMIA-REPERFUSION WISTAR RAT MODEL
I. Goicoechea, L.I. Peri, M.J. Vicent, M. Orzaez, L. Mengual, M. Solé, E. Pérez-Paya, A. Alcaraz
European Urology Supplements March 2009 Volume 8, Issue 4, Page 144 DOI: https://doi.org/10.1016/S1569-9056(09)60100-7
Introduction & Objectives: In the complex process of apoptosis activation, protein Apaf-1 and procaspase 9 play a key role, as their activation induces apoptotic cascade. Different substances have been created in order to inhibit apoptosis and diminish the harmful effects produced in organs who have suffered an ischemia-reperfusion injury. Our aim is to determine the antiapoptotic activity of a family of molecules derived from peptoid 1, an Apaf-1 inhibitor, in an ischemia-reperfusion Wistar rat model, so as to evaluate the possibility of using them in clinical practice, particularly in the field of kidney transplantation.
Material & Methods: Animals were distributed in five groups of eight individuals. Each group received one of the following drugs and dosification: 1 PBS (control); 2 DMSO (control); 3 QM56 70 mg/kg; 4 P-QM31 100mg/kg; 5 P-QM31 45mg/kg. Drug administration took place at first hour in the morning and rats were sacrificed 8 hours later. One kidney was removed after 30 minutes of warm ischaemia and the contralateral after 90 minutes. Variables taken into account for statistical analysis were: caspase 3 activity, number of apoptotic cells using TUNELstaining and histological changes.
Results: In fig A, we can see caspase 3 activity from kidney cell extracts. It is clear how C3 activity decreases in the presence of Apaf-1 inhibitors (Anova 0,0002, Kruskal-Wallis 0,0025). Number of apoptotic cells is shown in figure B. Rats that received an inhibitor had less number of apoptotic cells than their controls (Anova 0,0002).
Conclusions: P-QM31 and QM56 can inhibit apoptosis cascade by inhibiting Apaf-1 in an in vivo model. Further research should be done in order to evaluate their usefulness in clinical practice.
NOVEL ANTICANCER POLYMER CONJUGATES DESIGNED TO TREAT HORMONE- DEPENDANT CANCERS AND CIRCUMVENT RESISTANCE
R. Duncan , M. J. Vicent , F. Greco , H. R. P Gilbert , D. Schmaljohann , E. Ferguson
Proceedings of the Controlled Release Society Meeting in Pamplona 2006
Introduction: Over the last decade” polymer therapeutics” have emerged as firstgeneration nanomedicines. The ability of polymer anticancer-drug conjugates to decrease drug toxicity and improve antitumour activity is now well established pre-clinically and several conjugates show promise in clinical trials (1-4). So far those conjugates that have entered clinical development all carry well-known chemotherapeutic agents including doxorubicin (4), platinates (1) and paclitaxel (5). Designed for lysosomotropic delivery (Figure 1), their whole body and cellular pharmacokinetics brings advantages of passive tumour targeting due to the hyperpermeability of tumour vasculature, and the ability to bypass some resistance mechanisms (reviewed in (1)). Polymer-drug linkers are often designed for cleavage by lysosomal thiol-dependent proteases (eg cathepsin B) or the reduced pH of endosomes and lysosomes to facilitate intracellular drug liberation. It is becoming clear that inappropriate trafficking and/or malfunction of enzymatic activation can lead to new mechanisms of clinical resistance.
Polyacetal-DES as novel pH-triggered anticancer therapeutics.
Vicent, M., R. Tomlinson, S. Brocchini and R. Duncan (2004).
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY, AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA.
