Manuscripts
O. Zagorodko, T. Melnyk, O. Rogier, V.J. Nebot, M.J. Vicent, Higher-order interfiber interactions in the self-assembly of benzene-1,3,5-tricarboxamide-based peptides in water. Polymer Chemistry. 2021;12:3478-3487 [Free Download at Polymer Chemistry][Zenodo][PubMed]
 Mimicking the complexity of biological systems with synthetic supramolecular materials requires a deep understanding of the relationship between the structure of the molecule and its self-assembly pattern. Herein, we report a series of water-soluble benzene-1,3,5-tricarboxamide-based di- and tripeptide derivatives modified with small non-bulky terminal amine salt to induce self-assembly into twisted one-dimensional higher-order nanofibers. The morphology of nanofibers strongly depends on the nature, order, and quantity of amino acids in the short peptide fragments and vary from simple cylindrical to complex helical. From observations of several fiber-splitting events, we detected interfiber interactions that always occur in a pairwise manner, which implies that the C3 symmetry of benzene-1,3,5-tricarboxamide-based molecules in higher-order fibers becomes gradually distorted, thus facilitating hydrophobic contact interactions between fibrils. The proposed mechanism of self-assembly through hydrophobic contact allowed the successful design of a compound with pH-responsive morphology, and may find use in the future development of complex hierarchical architectures with controlled functionality.
Mimicking the complexity of biological systems with synthetic supramolecular materials requires a deep understanding of the relationship between the structure of the molecule and its self-assembly pattern. Herein, we report a series of water-soluble benzene-1,3,5-tricarboxamide-based di- and tripeptide derivatives modified with small non-bulky terminal amine salt to induce self-assembly into twisted one-dimensional higher-order nanofibers. The morphology of nanofibers strongly depends on the nature, order, and quantity of amino acids in the short peptide fragments and vary from simple cylindrical to complex helical. From observations of several fiber-splitting events, we detected interfiber interactions that always occur in a pairwise manner, which implies that the C3 symmetry of benzene-1,3,5-tricarboxamide-based molecules in higher-order fibers becomes gradually distorted, thus facilitating hydrophobic contact interactions between fibrils. The proposed mechanism of self-assembly through hydrophobic contact allowed the successful design of a compound with pH-responsive morphology, and may find use in the future development of complex hierarchical architectures with controlled functionality.
A. Duro-Castano, C. Borras, V. Herranz-Pérez, M. C. Blanco-Gandía, I. Conejos-Sánchez, A. Armiñán, C. Mas-Bargues, M. Inglés, J. Miñarro, M. Rodríguez-Arias, J. M. García-Verdugo, J. Viña, M. J. Vicent*. Targeting Alzheimer’s disease with multimodal polypeptide-based nanoconjugates. Science Advances 2021;7:eabf9180. [PubMed][Free Download at Science Advances][Zenodo]
 Alzheimer’s disease (AD), the most prevalent form of dementia, remains incurable mainly due to our failings in the search for effective pharmacological strategies. Here, we describe the development of targeted multimodal polypeptide-based nanoconjugates as potential AD treatments. Treatment with polypeptide nanoconjugates bearing propargylamine moieties and bisdemethoxycurcumin or genistein afforded neuroprotection and displayed neurotrophic effects, as evidenced by an increase in dendritic density of pyramidal neurons in organotypic hippocampal culture. The additional conjugation of the Angiopep-2 targeting moiety enhanced nanoconjugate passage through the blood-brain barrier and modulated brain distribution with nanoconjugate accumulation in neurogenic areas, including the olfactory bulb. Nanoconjugate treatment effectively reduced neurotoxic β amyloid aggregate levels and rescued impairments to olfactory memory and object recognition in APP/PS1 transgenic AD model mice. Overall, this study provides a description of a targeted multimodal polyglutamate-based nanoconjugate with neuroprotective and neurotrophic potential for AD treatment.
Alzheimer’s disease (AD), the most prevalent form of dementia, remains incurable mainly due to our failings in the search for effective pharmacological strategies. Here, we describe the development of targeted multimodal polypeptide-based nanoconjugates as potential AD treatments. Treatment with polypeptide nanoconjugates bearing propargylamine moieties and bisdemethoxycurcumin or genistein afforded neuroprotection and displayed neurotrophic effects, as evidenced by an increase in dendritic density of pyramidal neurons in organotypic hippocampal culture. The additional conjugation of the Angiopep-2 targeting moiety enhanced nanoconjugate passage through the blood-brain barrier and modulated brain distribution with nanoconjugate accumulation in neurogenic areas, including the olfactory bulb. Nanoconjugate treatment effectively reduced neurotoxic β amyloid aggregate levels and rescued impairments to olfactory memory and object recognition in APP/PS1 transgenic AD model mice. Overall, this study provides a description of a targeted multimodal polyglutamate-based nanoconjugate with neuroprotective and neurotrophic potential for AD treatment.
A. Duro-Castano, A. Sousa-Herves, A. Armiñán, D. Charbonnier. J.J. Arroyo-Crespo, S. Wedepohl, M. Calderón*, M. J. Vicent*. Polyglutamic acid-based crosslinked doxorubicin nanogels as an anti-metastatic treatment for triple negative breast cancer. J. Control. Rel. 2021;332:10-20. [Journal Site][PubMed][Repository Link][Front Page Image]
 Treatment of triple negative breast cancer (TNBC)-associated metastasis represents an unmet clinical need, and we lack effective therapeutics for a disease that exhibits high relapse rates and associates with poor patient outcomes. Advanced nanosized drug delivery systems may enhance the efficacy of first-line chemotherapeutics by altering drug pharmacokinetics and enhancing tumor/metastasis targeting to significantly improve efficacy and safety. Herein, we propose the application of injectable poly-amino acid-based nanogels (NGs) as a versatile hydrophilic drug delivery platform for the treatment of TNBC lung metastasis. We prepared biocompatible and biodegradable cross-linked NGs from polyglutamic acid (PGA) loaded with the chemotherapeutic agent doxorubicin (DOX). Our optimized synthetic procedures generated NGs of ~100 nm in size and 25 wt% drug loading content that became rapidly internalized in TNBC cell lines and displayed IC50 values comparable to the free form of DOX. Importantly, PGA-DOX NGs significantly inhibited lung metastases and almost completely suppressed lymph node metastases in a spontaneously metastatic orthotopic mouse TNBC model. Overall, our newly developed PGA-DOX NGs represent a potentially effective therapeutic strategy for the treatment of TNBC metastases.
Treatment of triple negative breast cancer (TNBC)-associated metastasis represents an unmet clinical need, and we lack effective therapeutics for a disease that exhibits high relapse rates and associates with poor patient outcomes. Advanced nanosized drug delivery systems may enhance the efficacy of first-line chemotherapeutics by altering drug pharmacokinetics and enhancing tumor/metastasis targeting to significantly improve efficacy and safety. Herein, we propose the application of injectable poly-amino acid-based nanogels (NGs) as a versatile hydrophilic drug delivery platform for the treatment of TNBC lung metastasis. We prepared biocompatible and biodegradable cross-linked NGs from polyglutamic acid (PGA) loaded with the chemotherapeutic agent doxorubicin (DOX). Our optimized synthetic procedures generated NGs of ~100 nm in size and 25 wt% drug loading content that became rapidly internalized in TNBC cell lines and displayed IC50 values comparable to the free form of DOX. Importantly, PGA-DOX NGs significantly inhibited lung metastases and almost completely suppressed lymph node metastases in a spontaneously metastatic orthotopic mouse TNBC model. Overall, our newly developed PGA-DOX NGs represent a potentially effective therapeutic strategy for the treatment of TNBC metastases.
D. Van Lysebetten, A. Malfanti, K. Deswarte, K. Koynov, B. Golba, T. Ye, Z. Zhong, S. Kasmi, A. Lamoot, Y. Chen, S. Van Herck, B. N. Lambrecht, N. N. Sanders, S. Lienenklaus, S. A. David, M. J. Vicent, S. De Koker, B. G. De Geest. Lipid-Polyglutamate Nanoparticle Vaccine Platform. ACS Applied Materials & Interfaces 2021;13:6011-6022. [PubMed][Journal Site][Repository Link]
 Peptide-based subunit vaccines are attractive in view of personalized cancer vaccination with neo-antigens, as well as for the design of the newest generation of vaccines against infectious diseases. Key to mounting robust antigen-specific immunity is delivery of antigen to antigen-presenting (innate immune) cells in lymphoid tissue with concomitant innate immune activation to promote antigen presentation to T cells and to shape the amplitude and nature of the immune response. Nanoparticles that co-deliver both peptide antigen and molecular adjuvants are well suited for this task. However, in the context of peptide-based antigen, an unmet need exists for a generic strategy that allows for co-encapsulation of peptide and molecular adjuvants due to the stark variation in physicochemical properties based on the amino acid sequence of the peptide. These properties also strongly differ from those of many molecular adjuvants. Here, we devise a lipid nanoparticle (LNP) platform that addresses these issues. Key in our concept is poly(l-glutamic acid) (PGA), which serves as a hydrophilic backbone for conjugation of, respectively, peptide antigen (Ag) and an imidazoquinoline (IMDQ) TLR7/8 agonist as a molecular adjuvant. Making use of the PGA’s polyanionic nature, we condensate PGA-Ag and PGA-IMDQ into LNP by electrostatic interaction with an ionizable lipid. We show in vitro and in vivo in mouse models that LNP encapsulation favors uptake by innate immune cells in lymphoid tissue and promotes the induction of Ag-specific T cells responses both after subcutaneous and intravenous administration.
Peptide-based subunit vaccines are attractive in view of personalized cancer vaccination with neo-antigens, as well as for the design of the newest generation of vaccines against infectious diseases. Key to mounting robust antigen-specific immunity is delivery of antigen to antigen-presenting (innate immune) cells in lymphoid tissue with concomitant innate immune activation to promote antigen presentation to T cells and to shape the amplitude and nature of the immune response. Nanoparticles that co-deliver both peptide antigen and molecular adjuvants are well suited for this task. However, in the context of peptide-based antigen, an unmet need exists for a generic strategy that allows for co-encapsulation of peptide and molecular adjuvants due to the stark variation in physicochemical properties based on the amino acid sequence of the peptide. These properties also strongly differ from those of many molecular adjuvants. Here, we devise a lipid nanoparticle (LNP) platform that addresses these issues. Key in our concept is poly(l-glutamic acid) (PGA), which serves as a hydrophilic backbone for conjugation of, respectively, peptide antigen (Ag) and an imidazoquinoline (IMDQ) TLR7/8 agonist as a molecular adjuvant. Making use of the PGA’s polyanionic nature, we condensate PGA-Ag and PGA-IMDQ into LNP by electrostatic interaction with an ionizable lipid. We show in vitro and in vivo in mouse models that LNP encapsulation favors uptake by innate immune cells in lymphoid tissue and promotes the induction of Ag-specific T cells responses both after subcutaneous and intravenous administration.
O. Zagorodko, V. J. Nebot*, and M. J. Vicent*. The generation of stabilized supramolecular nanorods from star-shaped polyglutamates. Polymer Chemistry, 2020(11):1220-1229. [Journal Site][Zenodo]
 We developed a new strategy of polyglutamate nanorod preparation based on supramolecular polymers stabilized with hydrophobic drugs. Using this strategy, we prepared a family of star-shaped polyglutamates (star-PGAs) with benzenetricarboxamide (BTA)-based cores of different hydrophobicity. We then studied the self-assembly of the resulting polymers in aqueous solutions containing a physiological level of salt using fluorescence spectroscopy, small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). We discovered that star-PGAs behave as classical polyelectrolytes in very dilute solutions; however, compounds with hydrophobic cores assembled into one dimensional-nanorods upon an increase in concentration due to supramolecular interactions in the core. Small hydrophobic drugs, such as doxorubicin and irinotecan, stabilized the nanorods and inhibited their disassembly at concentrations below the critical aggregation concentration (CAC). We anticipate that this simple nanorod preparation strategy from star-PGAs will enable the development of new nanomedicines with unique biodistribution profiles and biological activity.
We developed a new strategy of polyglutamate nanorod preparation based on supramolecular polymers stabilized with hydrophobic drugs. Using this strategy, we prepared a family of star-shaped polyglutamates (star-PGAs) with benzenetricarboxamide (BTA)-based cores of different hydrophobicity. We then studied the self-assembly of the resulting polymers in aqueous solutions containing a physiological level of salt using fluorescence spectroscopy, small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). We discovered that star-PGAs behave as classical polyelectrolytes in very dilute solutions; however, compounds with hydrophobic cores assembled into one dimensional-nanorods upon an increase in concentration due to supramolecular interactions in the core. Small hydrophobic drugs, such as doxorubicin and irinotecan, stabilized the nanorods and inhibited their disassembly at concentrations below the critical aggregation concentration (CAC). We anticipate that this simple nanorod preparation strategy from star-PGAs will enable the development of new nanomedicines with unique biodistribution profiles and biological activity.
Arroyo‐Crespo, J. J., Armiñán, A.* , Charbonnier, D. , Deladriere, C. , Palomino‐Schätzlein, M. , Lamas‐Domingo, R. , Forteza, J. , Pineda‐Lucena, A. and Vicent, M. J.* Characterization of Triple‐Negative Breast Cancer Preclinical Models Provides Functional Evidence of Metastatic Progression. International Journal of Cancer, 2019;145(8):2267-2281. [PubMed][Journal Site][Zenodo]
 Triple‐negative breast cancer (TNBC), an aggressive, metastatic, and recurrent breast cancer (BC) subtype, currently suffers from a lack of adequately described spontaneously metastatic preclinical models that faithfully reproduce the clinical scenario. We describe two preclinical spontaneously metastatic TNBC orthotopic murine models for the development of advanced therapeutics: an immunodeficient human MDA‐MB‐231‐Luc model and an immunocompetent mouse 4T1 model. Furthermore, we provide a broad range of multifactorial analysis for both models that could provide relevant information for the development of new therapies and diagnostic tools. Our comparisons uncovered differential growth rates, stromal arrangements, and metabolic profiles in primary tumors, and the presence of cancer‐associated adipocyte infiltration in the MDA‐MB‐231‐Luc model. Histopathological studies highlighted the more rapid metastatic spread to the lungs in the 4T1 model following a lymphatic route, while we observed both homogeneous (MDA‐MB‐231‐Luc) and heterogeneous (4T1) metastatic spread to axillary lymph nodes. We encountered unique metabolomic signatures in each model, including crucial amino acids and cell membrane components. Hematological analysis demonstrated severe leukemoid and lymphoid reactions in the 4T1 model with the partial reestablishment of immune responses in the immunocompromised MDA‐MB‐231‐Luc model. Additionally, we discovered β‐immunoglobulinemia and increased basal levels of G‐CSF correlating with a metastatic switch, with G‐CSF also promoting extramedullary hematopoiesis (both models) and causing hepatosplenomegaly (4T1 model). Overall, we believe that the characterization of these preclinical models will foster the development of advanced therapeutic strategies for TNBC treatment, especially for the treatment of patients presenting both, primary tumors and metastatic spread.
Triple‐negative breast cancer (TNBC), an aggressive, metastatic, and recurrent breast cancer (BC) subtype, currently suffers from a lack of adequately described spontaneously metastatic preclinical models that faithfully reproduce the clinical scenario. We describe two preclinical spontaneously metastatic TNBC orthotopic murine models for the development of advanced therapeutics: an immunodeficient human MDA‐MB‐231‐Luc model and an immunocompetent mouse 4T1 model. Furthermore, we provide a broad range of multifactorial analysis for both models that could provide relevant information for the development of new therapies and diagnostic tools. Our comparisons uncovered differential growth rates, stromal arrangements, and metabolic profiles in primary tumors, and the presence of cancer‐associated adipocyte infiltration in the MDA‐MB‐231‐Luc model. Histopathological studies highlighted the more rapid metastatic spread to the lungs in the 4T1 model following a lymphatic route, while we observed both homogeneous (MDA‐MB‐231‐Luc) and heterogeneous (4T1) metastatic spread to axillary lymph nodes. We encountered unique metabolomic signatures in each model, including crucial amino acids and cell membrane components. Hematological analysis demonstrated severe leukemoid and lymphoid reactions in the 4T1 model with the partial reestablishment of immune responses in the immunocompromised MDA‐MB‐231‐Luc model. Additionally, we discovered β‐immunoglobulinemia and increased basal levels of G‐CSF correlating with a metastatic switch, with G‐CSF also promoting extramedullary hematopoiesis (both models) and causing hepatosplenomegaly (4T1 model). Overall, we believe that the characterization of these preclinical models will foster the development of advanced therapeutic strategies for TNBC treatment, especially for the treatment of patients presenting both, primary tumors and metastatic spread.
Arroyo-Crespo, J.J., Armiñán, A., Charbonnier, D., Balzano-Nogueira, L., Huertas-López, F., Martí, C., Tarazona, S., Forteza, J., Conesa, A., Vicent, M.J*. Tumor microenvironment-targeted poly-L-glutamic acid-based combination conjugate for enhanced triple negative breast cancer treatment. Biomaterials, 2018. 186: p8-21. [PubMed][Free Download at Biomaterials][Zenodo].
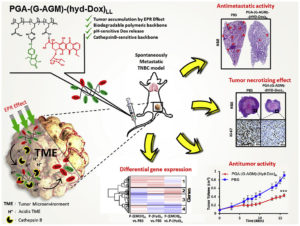 The intrinsic characteristics of the tumor microenvironment (TME), including acidic pH and overexpression of hydrolytic enzymes, offer an exciting opportunity for the rational design of TME-drug delivery systems (DDS). We developed and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-based combination conjugate family with the aim of optimizing anticancer effects. We obtained combination conjugates bearing Doxorubicin (Dox) and aminoglutethimide (AGM) with two Dox loadings and two different hydrazone pH-sensitive linkers that promote the specific release of Dox from the polymeric backbone within the TME. Low Dox loading coupled with a short hydrazone linker yielded optimal effects on primary tumor growth, lung metastasis (∼90% reduction), and toxicological profile in a preclinical metastatic triple-negative breast cancer (TNBC) murine model. The use of transcriptomic analysis helped us to identify the molecular mechanisms responsible for such results including a differential immunomodulation and cell death pathways among the conjugates. This data highlights the advantages of targeting the TME, the therapeutic value of polymer-based combination approaches, and the utility of –omics-based analysis to accelerate anticancer DDS.
The intrinsic characteristics of the tumor microenvironment (TME), including acidic pH and overexpression of hydrolytic enzymes, offer an exciting opportunity for the rational design of TME-drug delivery systems (DDS). We developed and characterized a pH-responsive biodegradable poly-L-glutamic acid (PGA)-based combination conjugate family with the aim of optimizing anticancer effects. We obtained combination conjugates bearing Doxorubicin (Dox) and aminoglutethimide (AGM) with two Dox loadings and two different hydrazone pH-sensitive linkers that promote the specific release of Dox from the polymeric backbone within the TME. Low Dox loading coupled with a short hydrazone linker yielded optimal effects on primary tumor growth, lung metastasis (∼90% reduction), and toxicological profile in a preclinical metastatic triple-negative breast cancer (TNBC) murine model. The use of transcriptomic analysis helped us to identify the molecular mechanisms responsible for such results including a differential immunomodulation and cell death pathways among the conjugates. This data highlights the advantages of targeting the TME, the therapeutic value of polymer-based combination approaches, and the utility of –omics-based analysis to accelerate anticancer DDS.
Arroyo‐Crespo, J.J., Deladriere, C., Nebot, V.J., Charbonnier, D., Masiá, E., Paul, A., James, C., Armiñán, A.*, and Vicent, M.J.*, Anticancer Activity Driven by Drug Linker Modification in a Polyglutamic Acid‐Based Combination‐Drug Conjugate. Advanced Functional Materials, 2018. 28(22): p. 1800931 [See Full text free at Advanced Functional Materials][Zenodo]
 Combination nanotherapies for the treatment of breast cancer permits synergistic drug targeting of multiple pathways. However, poor carrier degradability, poor synergism of the combined drugs, low drug release regulation, and a lack of control on final macromolecule solution conformation (which drives the biological fate) limit the application of this strategy. The present study describes the development of a family of drug delivery systems composed of chemotherapeutic (doxorubicin) and endocrine therapy (aromatase inhibitor aminoglutethimide) agents conjugated to a biodegradable poly‐l‐glutamic acid backbone via various linking moieties. Data from in vitro cytotoxicity and drug release assessments and animal model validation select a conjugate family member with optimal biological performance. Exhaustive physicochemical characterization in relevant media (including the study of secondary structure, size measurements, and detailed small‐angle neutron scattering analysis) correlates biological data with the intrinsic supramolecular characteristics of the conjugate. Overall, this study demonstrates how a small flexible Gly linker can modify the spatial conformation of the entire polymer-drug conjugate, promote the synergistic release of both drugs, and significantly improve biological activity. These findings highlight the need for a deeper understanding of polymer-drug conjugates at the supramolecular level to allow the design of more effective polymer-drug conjugates.
Combination nanotherapies for the treatment of breast cancer permits synergistic drug targeting of multiple pathways. However, poor carrier degradability, poor synergism of the combined drugs, low drug release regulation, and a lack of control on final macromolecule solution conformation (which drives the biological fate) limit the application of this strategy. The present study describes the development of a family of drug delivery systems composed of chemotherapeutic (doxorubicin) and endocrine therapy (aromatase inhibitor aminoglutethimide) agents conjugated to a biodegradable poly‐l‐glutamic acid backbone via various linking moieties. Data from in vitro cytotoxicity and drug release assessments and animal model validation select a conjugate family member with optimal biological performance. Exhaustive physicochemical characterization in relevant media (including the study of secondary structure, size measurements, and detailed small‐angle neutron scattering analysis) correlates biological data with the intrinsic supramolecular characteristics of the conjugate. Overall, this study demonstrates how a small flexible Gly linker can modify the spatial conformation of the entire polymer-drug conjugate, promote the synergistic release of both drugs, and significantly improve biological activity. These findings highlight the need for a deeper understanding of polymer-drug conjugates at the supramolecular level to allow the design of more effective polymer-drug conjugates.
Armiñán, A., Palomino-Schätzlein, M., Deladriere, C., Arroyo-Crespo, J. J., Vicente-Ruiz, S., Vicent, M. J.*, and Pineda-Lucena, A.* Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials, 2018. 162: p144-153. [PubMed][Zenodo][Download PDF free at Biomaterials]
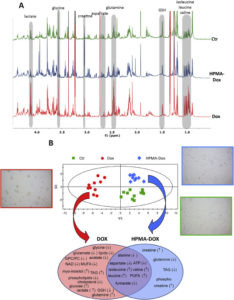 Metabolomics is becoming a relevant tool for understanding the molecular mechanisms involved in the response to new drug delivery systems. The applicability of this experimental approach to cell cultures and animal models makes metabolomics a useful tool for establishing direct connections between in vitro and in vivo data, thus providing a reliable platform for the characterization of chemotherapeutic agents. Herein, we used metabolomic profiles based on nuclear magnetic resonance (NMR) spectroscopy to evaluate the biochemical pathways involved in the response to a chemotherapeutic anthracycline drug (Doxorubicin, Dox) and an N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-conjugated form (HPMA-Dox) in an in vitro cell culture model and an in vivo orthotopic breast cancer model. We also used protein expression and flow cytometry studies to obtain a better coverage of the biochemical alterations associated with the administration of these compounds. The overall analysis revealed that polymer conjugation leads to increased apoptosis, reduced glycolysis, and reduced levels of phospholipids when compared to the free chemotherapeutic drug. Our results represent a first step in the application of integrated in vitro and in vivo metabolomic studies to the evaluation of drug delivery systems.
Metabolomics is becoming a relevant tool for understanding the molecular mechanisms involved in the response to new drug delivery systems. The applicability of this experimental approach to cell cultures and animal models makes metabolomics a useful tool for establishing direct connections between in vitro and in vivo data, thus providing a reliable platform for the characterization of chemotherapeutic agents. Herein, we used metabolomic profiles based on nuclear magnetic resonance (NMR) spectroscopy to evaluate the biochemical pathways involved in the response to a chemotherapeutic anthracycline drug (Doxorubicin, Dox) and an N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-conjugated form (HPMA-Dox) in an in vitro cell culture model and an in vivo orthotopic breast cancer model. We also used protein expression and flow cytometry studies to obtain a better coverage of the biochemical alterations associated with the administration of these compounds. The overall analysis revealed that polymer conjugation leads to increased apoptosis, reduced glycolysis, and reduced levels of phospholipids when compared to the free chemotherapeutic drug. Our results represent a first step in the application of integrated in vitro and in vivo metabolomic studies to the evaluation of drug delivery systems.
Duro-Castaño, A., Nebot, V. J., Niño-Pariente, A., Armiñán, A., Arroyo-Crespo, J. J., Paul, A., Feiner-Gracia, N., Albertazzi, L. and Vicent, M. J.* Capturing “Extraordinary” Soft-Assembled Charge-Like Polypeptides as a Strategy for Nanocarrier Design. Adv Mater, 2017. 29(39): p. 1702888-n/a. [PubMed] [Zenodo][Download PDF free at Advanced Materials]
 The rational design of nanomedicines is a challenging task given the complex architectures required for the construction of nanosized carriers with embedded therapeutic properties and the complex interface of these materials with the biological environment. Herein, an unexpected charge-like attraction mechanism of self-assembly for star-shaped polyglutamates in nonsalty aqueous solutions is identified, which matches the ubiquitous “ordinary-extraordinary” phenomenon previously described by physicists. For the first time, a bottom-up methodology for the stabilization of these nanosized soft-assembled star-shaped polyglutamates is also described, enabling the translation of theoretical research into nanomaterials with applicability within the drug-delivery field. Covalent capture of these labile assemblies provides access to unprecedented architectures to be used as nanocarriers. The enhanced in vitro and in vivo properties of these novel nanoconstructs as drug-delivery systems highlight the potential of this approach for tumor-localized as well as lymphotropic delivery.
The rational design of nanomedicines is a challenging task given the complex architectures required for the construction of nanosized carriers with embedded therapeutic properties and the complex interface of these materials with the biological environment. Herein, an unexpected charge-like attraction mechanism of self-assembly for star-shaped polyglutamates in nonsalty aqueous solutions is identified, which matches the ubiquitous “ordinary-extraordinary” phenomenon previously described by physicists. For the first time, a bottom-up methodology for the stabilization of these nanosized soft-assembled star-shaped polyglutamates is also described, enabling the translation of theoretical research into nanomaterials with applicability within the drug-delivery field. Covalent capture of these labile assemblies provides access to unprecedented architectures to be used as nanocarriers. The enhanced in vitro and in vivo properties of these novel nanoconstructs as drug-delivery systems highlight the potential of this approach for tumor-localized as well as lymphotropic delivery.
Review Articles
Boix-Montesinos P., Soriano-Teruel P. M., Armiñán A., Orzáez, M, & Vicent M. J. The Past, Present, and Future of Breast Cancer Models for Nanomedicine Development. Advanced Drug Delivery Reviews 2021;173:306-330. [PubMed][Journal Website][Zenodo]
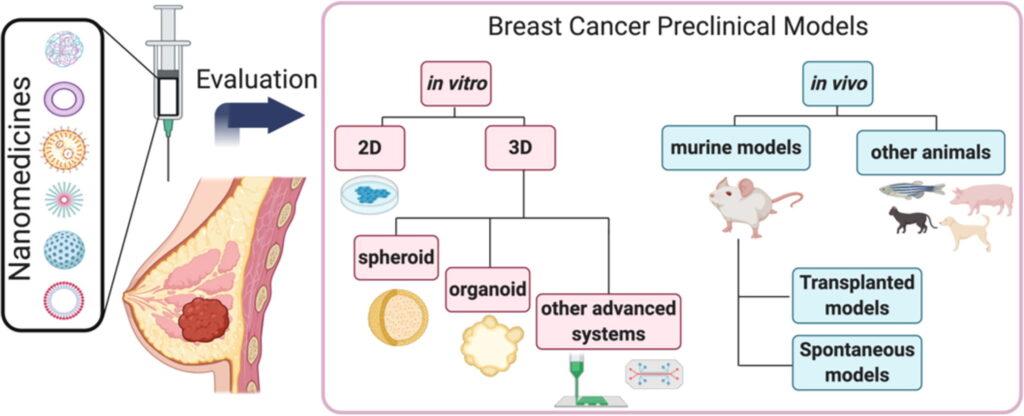 Even given recent advances in nanomedicine development of breast cancer treatment in recent years and promising results in pre-clinical models, cancer nanomedicines often fail at the clinical trial stage. Limitations of conventional in vitro models include the lack of representation of the stromal population, the absence of a three-dimensional (3D) structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. Herein, we review those cell culture strategies that aim to overcome these limitations, including cell co-cultures, advanced 3D cell cultures, patient-derived cells, bioprinting, and microfluidics systems. The in vivo evaluation of nanomedicines must consider critical parameters that include the enhanced permeability and retention effect, the host’s immune status, and the site of tumor implantation. Here, we critically discuss the advantages and limitations of current in vivo models and report how the improved selection and application of breast cancer models can improve the clinical translation of nanomedicines.
Even given recent advances in nanomedicine development of breast cancer treatment in recent years and promising results in pre-clinical models, cancer nanomedicines often fail at the clinical trial stage. Limitations of conventional in vitro models include the lack of representation of the stromal population, the absence of a three-dimensional (3D) structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. Herein, we review those cell culture strategies that aim to overcome these limitations, including cell co-cultures, advanced 3D cell cultures, patient-derived cells, bioprinting, and microfluidics systems. The in vivo evaluation of nanomedicines must consider critical parameters that include the enhanced permeability and retention effect, the host’s immune status, and the site of tumor implantation. Here, we critically discuss the advantages and limitations of current in vivo models and report how the improved selection and application of breast cancer models can improve the clinical translation of nanomedicines.
Melnyk, T., Đorđević, S., Conejos-Sánchez, I., and Vicent, M. J. Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation. Advanced Drug Delivery Reviews 2020; 160: 136-169. [Journal Website][Zenodo][PubMed]
 The clinical success of polypeptides as polymeric drugs, covered by the umbrella term “polymer therapeutics,” combined with related scientific and technological breakthroughs, explain their exponential growth in the development of polypeptide-drug conjugates as therapeutic agents. A deeper understanding of the biology at relevant pathological sites and the critical biological barriers faced, combined with advances regarding controlled polymerization techniques, material bioresponsiveness, analytical methods, and scale up-manufacture processes, have fostered the development of these nature-mimicking entities. Now, engineered polypeptides have the potential to combat current challenges in the advanced drug delivery field. In this review, we will discuss examples of polypeptide-drug conjugates as single or combination therapies in both preclinical and clinical studies as therapeutics and molecular imaging tools. Importantly, we will critically discuss relevant examples to highlight those parameters relevant to their rational design, such as linking chemistry, the analytical strategies employed, and their physicochemical and biological characterization, that will foster their rapid clinical translation.
The clinical success of polypeptides as polymeric drugs, covered by the umbrella term “polymer therapeutics,” combined with related scientific and technological breakthroughs, explain their exponential growth in the development of polypeptide-drug conjugates as therapeutic agents. A deeper understanding of the biology at relevant pathological sites and the critical biological barriers faced, combined with advances regarding controlled polymerization techniques, material bioresponsiveness, analytical methods, and scale up-manufacture processes, have fostered the development of these nature-mimicking entities. Now, engineered polypeptides have the potential to combat current challenges in the advanced drug delivery field. In this review, we will discuss examples of polypeptide-drug conjugates as single or combination therapies in both preclinical and clinical studies as therapeutics and molecular imaging tools. Importantly, we will critically discuss relevant examples to highlight those parameters relevant to their rational design, such as linking chemistry, the analytical strategies employed, and their physicochemical and biological characterization, that will foster their rapid clinical translation.
Moura, LIF., Malfanti, A., Peres, C., Matos, AI., Guegain, E., Sainz, W., Zloh, Vicent, MJ., Florindo, HF. Functionalized Branched Polymers: Promising Immunomodulatory Tools for the Treatment of Cancer and Immune Disorders. Materials Horizons, 2019;6:1956-1973 [Journal Website][Zenodo]
 Well-defined synthetic branched nanostructures form an emerging subclass of macromolecular structures, whose 3D structure and multivalency offer unique opportunities for fine-tuning their internalization and cellular targeting. In particular, dendrimers possess a well-defined 3D-globular backbone with highly versatile functional surface groups and exhibit a range of chemical and biological properties. Branched polymers present unique opportunities for the targeted delivery of diverse bioactive molecules (including targeting ligands, imaging probes, and drugs) via conjugation to multiple sites within the structure. The inherent versatility and multifunctionality of these architectures make them potentially useful for the modulation of multiple immune-related pathways for the treatment of a wide range of disease and disorders, including cancer and human immunodeficiency virus infection. Herein, we describe the key components of the immune system whose targeting can help to overcome immune-related disorders and discuss branched polymers (including dendrimers) as promising delivery systems with unique immunomodulatory properties against cancer and infectious diseases.
Well-defined synthetic branched nanostructures form an emerging subclass of macromolecular structures, whose 3D structure and multivalency offer unique opportunities for fine-tuning their internalization and cellular targeting. In particular, dendrimers possess a well-defined 3D-globular backbone with highly versatile functional surface groups and exhibit a range of chemical and biological properties. Branched polymers present unique opportunities for the targeted delivery of diverse bioactive molecules (including targeting ligands, imaging probes, and drugs) via conjugation to multiple sites within the structure. The inherent versatility and multifunctionality of these architectures make them potentially useful for the modulation of multiple immune-related pathways for the treatment of a wide range of disease and disorders, including cancer and human immunodeficiency virus infection. Herein, we describe the key components of the immune system whose targeting can help to overcome immune-related disorders and discuss branched polymers (including dendrimers) as promising delivery systems with unique immunomodulatory properties against cancer and infectious diseases.
Rodriguez‐Otormin, F., Duro‐Castaño, A., Conejos‐Sánchez, I., Vicent, M.J. Envisioning the Future of Polymer Therapeutics for Brain Disorders. WIREs Nanomedicine and Nanobiotechnology, 2019;11:e1532. [PubMed][Journal Website][Zenodo][Advanced Science News][Inside Cover Image]
 The growing incidence of brain‐related pathologies and the problems that undermine the development of efficient and effective treatments have prompted both researchers and the pharmaceutical industry to search for novel therapeutic alternatives. Polymer therapeutics (PT) display properties well suited to the treatment of neuro‐related disorders, which help to overcome the many hidden obstacles on the journey to the central nervous system (CNS). The inherent features of PT, derived from drug(s) conjugation, in parallel with the progress in synthesis and analytical methods, the increasing knowledge in molecular basis of diseases, and collected clinical data through the last four decades, have driven the translation from “bench to bedside” for various biomedical applications. However, since the approval of Gliadel® wafers, little progress has been made in the CNS field, even though brain targeting represents an ever‐growing challenge. A thorough assessment of the steps required for successful brain delivery via different administration routes and the consideration of the disease‐specific hallmarks are essential to progress in the field. Within this review, we hope to summarize the latest developments, successes, and failures and discuss considerations on designs and strategies for PT in the treatment of CNS disorders.
The growing incidence of brain‐related pathologies and the problems that undermine the development of efficient and effective treatments have prompted both researchers and the pharmaceutical industry to search for novel therapeutic alternatives. Polymer therapeutics (PT) display properties well suited to the treatment of neuro‐related disorders, which help to overcome the many hidden obstacles on the journey to the central nervous system (CNS). The inherent features of PT, derived from drug(s) conjugation, in parallel with the progress in synthesis and analytical methods, the increasing knowledge in molecular basis of diseases, and collected clinical data through the last four decades, have driven the translation from “bench to bedside” for various biomedical applications. However, since the approval of Gliadel® wafers, little progress has been made in the CNS field, even though brain targeting represents an ever‐growing challenge. A thorough assessment of the steps required for successful brain delivery via different administration routes and the consideration of the disease‐specific hallmarks are essential to progress in the field. Within this review, we hope to summarize the latest developments, successes, and failures and discuss considerations on designs and strategies for PT in the treatment of CNS disorders.
Atkinson, S.P., Andreu, Z., and Vicent, M.J., Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. Journal of Personalized Medicine, 2018. 8(1): p. 6. [PubMed] [Free PDF Download at JPM][Zenodo]
 Polymer therapeutics (PTs) provides a potentially exciting approach for the treatment of many diseases by enhancing aqueous solubility and altering drug pharmacokinetics at both the whole organism and subcellular level leading to improved therapeutic outcomes. However, the failure of many polymer-drug conjugates in clinical trials suggests that we may need to stratify patients in order to match each patient to the right PT. In this concise review, we hope to assess potential PT-specific biomarkers for cancer treatment, with a focus on new studies, detection methods, new models and the opportunities this knowledge will bring for the development of novel PT-based anti-cancer strategies. We discuss the various “hurdles” that a given PT faces on its passage from the syringe to the tumor (and beyond), including the passage through the bloodstream, tumor targeting, tumor uptake and the intracellular release of the active agent. However, we also discuss other relevant concepts and new considerations in the field, which we hope will provide new insight into the possible applications of PT-related biomarkers.
Polymer therapeutics (PTs) provides a potentially exciting approach for the treatment of many diseases by enhancing aqueous solubility and altering drug pharmacokinetics at both the whole organism and subcellular level leading to improved therapeutic outcomes. However, the failure of many polymer-drug conjugates in clinical trials suggests that we may need to stratify patients in order to match each patient to the right PT. In this concise review, we hope to assess potential PT-specific biomarkers for cancer treatment, with a focus on new studies, detection methods, new models and the opportunities this knowledge will bring for the development of novel PT-based anti-cancer strategies. We discuss the various “hurdles” that a given PT faces on its passage from the syringe to the tumor (and beyond), including the passage through the bloodstream, tumor targeting, tumor uptake and the intracellular release of the active agent. However, we also discuss other relevant concepts and new considerations in the field, which we hope will provide new insight into the possible applications of PT-related biomarkers.
Duro-Castano, A., Gallon, E., Decker, C., and Vicent, M.J.*, Modulating Angiogenesis with Integrin-Targeted Nanomedicines. Adv Drug Deliv Rev, 2017. 119(Supplement C): p. 101-119. [PubMed] [Zenodo]
 Targeting angiogenesis-related pathologies, which include tumorigenesis and metastatic processes, has become an attractive strategy for the development of efficient guided nanomedicines. In this respect, integrins are cell-adhesion molecules involved in angiogenesis signaling pathways and are overexpressed in many angiogenic processes. Therefore, they represent specific biomarkers not only to monitor disease progression but also to rationally design targeted nanomedicines. Arginine-glycine-aspartic (RGD) containing peptides that bind to specific integrins have been widely utilized to provide ligand-mediated targeting capabilities to small molecules, peptides, proteins, and antibodies, as well as to drug/imaging agent-containing nanomedicines, with the final aim of maximizing their therapeutic index. Within this review, we aim to cover recent and relevant examples of different integrin-assisted nanosystems including polymeric nanoconstructs, liposomes, and inorganic nanoparticles applied in drug/gene therapy as well as imaging and theranostics. We will also critically address the overall benefits of integrin-targeting.
Targeting angiogenesis-related pathologies, which include tumorigenesis and metastatic processes, has become an attractive strategy for the development of efficient guided nanomedicines. In this respect, integrins are cell-adhesion molecules involved in angiogenesis signaling pathways and are overexpressed in many angiogenic processes. Therefore, they represent specific biomarkers not only to monitor disease progression but also to rationally design targeted nanomedicines. Arginine-glycine-aspartic (RGD) containing peptides that bind to specific integrins have been widely utilized to provide ligand-mediated targeting capabilities to small molecules, peptides, proteins, and antibodies, as well as to drug/imaging agent-containing nanomedicines, with the final aim of maximizing their therapeutic index. Within this review, we aim to cover recent and relevant examples of different integrin-assisted nanosystems including polymeric nanoconstructs, liposomes, and inorganic nanoparticles applied in drug/gene therapy as well as imaging and theranostics. We will also critically address the overall benefits of integrin-targeting.
Zagorodko, O., J. J. Arroyo-Crespo, V. J. Nebot and M. J. Vicent (2016). Polypeptide-Based Conjugates as Therapeutics: Opportunities and Challenges. Macromolecular Bioscience. 2017. 17(1). [PubMed] [Zenodo]
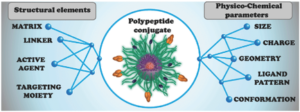 Synthetic polypeptides or polyamino acids have become a useful and multifunctional platform in advanced drug delivery studies. Nonetheless, the full potential of these systems has yet to be achieved. The final structure of polypeptide conjugates and their in vivo behavior are dependent on an extraordinarily complex pattern of interconnected physico-chemical and structural parameters, making sophisticated directional design of such systems difficult and often unachievable. In this review, the authors aim to discuss the role of these parameters in the successful design of different drug delivery architectures and to delineate some basic correlations between structure, properties, and the biological behavior of polypeptide-based conjugates.
Synthetic polypeptides or polyamino acids have become a useful and multifunctional platform in advanced drug delivery studies. Nonetheless, the full potential of these systems has yet to be achieved. The final structure of polypeptide conjugates and their in vivo behavior are dependent on an extraordinarily complex pattern of interconnected physico-chemical and structural parameters, making sophisticated directional design of such systems difficult and often unachievable. In this review, the authors aim to discuss the role of these parameters in the successful design of different drug delivery architectures and to delineate some basic correlations between structure, properties, and the biological behavior of polypeptide-based conjugates.
This review article was one of the journal’s top 20 most downloaded recent papers among articles published between July 2016 and June 2018.
Niño-Pariente, A., Nebot, V. J. and Vicent, M. J. (2016). Relevant Physicochemical Descriptors of “Soft Nanomedicines” to Bypass Biological Barriers. Current Pharmaceutical Design 22(9): 1274-1291. [PubMed] [Zenodo]
Herein, we present an overview on the current status of the characterization techniques and methodologies used to study the physicochemical descriptors that influence the final clinical performance of a given nanomedicine. The described techniques were selected based on their suitability to operate under relevant “native” conditions that mimic the physiological environment. Special emphasis is placed on those techniques that hold a greater potential to unravel dynamic, structural, and compositional features of soft organic nanomedicines relevant to the ability to bypass biological barriers, and hence allow for the rational design of drug delivery platforms with improved biological output.
Duro-Castano, A., Movellan, J., and Vicent, M.J.*, Smart branched polymer drug conjugates as nano-sized drug delivery systems. Biomater Sci, 2015. 3(10): p. 1321-34. [PubMed] [Zenodo]
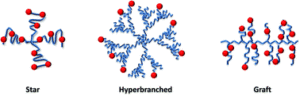 Polymer-drug conjugates represent excellent nanopharmaceutical candidates, as they offer multiple advantages related to their intrinsic characteristics. Many of the said characteristics are provided by the covalent bonding between the drug and the polymer. However, their clinical development has been slow and only one polymer-drug conjugate has reached the market, thus there remains an urgent need for the development of new and smart polymeric systems. Desirable characteristics of these new systems include higher molecular weight and degree of homogeneity, predictable conformations in solution, multivalency, and increased drug loading capacity, amongst others. With these aims in mind, branched polymers are ideal candidates due to their unique rheological, mechanical, and biomedical properties derived from their structure, inaccessible for linear polymers. Within this review, the synthetic strategies developed and the main efforts towards branched polymer implementation as carriers for polymer-drug conjugates will be addressed.
Polymer-drug conjugates represent excellent nanopharmaceutical candidates, as they offer multiple advantages related to their intrinsic characteristics. Many of the said characteristics are provided by the covalent bonding between the drug and the polymer. However, their clinical development has been slow and only one polymer-drug conjugate has reached the market, thus there remains an urgent need for the development of new and smart polymeric systems. Desirable characteristics of these new systems include higher molecular weight and degree of homogeneity, predictable conformations in solution, multivalency, and increased drug loading capacity, amongst others. With these aims in mind, branched polymers are ideal candidates due to their unique rheological, mechanical, and biomedical properties derived from their structure, inaccessible for linear polymers. Within this review, the synthetic strategies developed and the main efforts towards branched polymer implementation as carriers for polymer-drug conjugates will be addressed.
Awards
See the following link for all the information regarding Aroa Duro-Castano, who won an “Extraordinary Ph.D. Award” from the Universitat de València for her thesis, which made crucial contributions to the ERC MyNano project.
Videos
“Cross-linked Star-shaped self-assembled polyglutamates as vehicles in biomedical applications“
Book Chapters
Maso, K., Grigoletto, A., Vicent, M. J.*, & Pasut, G*. Molecular Platforms for Targeted Drug Delivery. In International Review of Cell and Molecular Biology: Academic Press, 2019.
Download from the repository here!
Patent Applications
PCT application number PCT/EP2016/067554
Title CROSS-LINKED STAR-SHAPED SELF-ASSEMBLED POLYPEPTIDES AND ITS USE AS CARRIERS IN BIOMEDICAL APPLICATIONS
Published Abstracts
AACR Annual Meeting 2018; April 14-18, 2018; Chicago, IL, USA – Abstract 3726: Design of personalised polymer based combination therapeutics for advanced stage breast cancer patients [Link]
NanoBio&Med 2018 November 20 – 22, Barcelona (Spain): Targeting Tumor Microenvironment with Rationally Designed Polypeptide-based Conjugates [PDF]
ICONAN2019 October 16-18, Munich (Germany): Polypeptide-based Conjugates as Versatile Therapeutics [PDF]
News Items
España, líder en la última convocatoria de ciencia europea [La Vanguardia]
Siete de los cincuenta científicos financiados por el Consejo Europeo de Investigación (ERC) en su última convocatoria de Pruebas de Concepto trabajan en instituciones españolas. Estas convocatorias están destinadas a explorar oportunidades de negocio a partir de investigaciones científicas de excelencia. España se sitúa, con el Reino Unido, como el país con más proyectos financiados. Cuatro de los siete son del Barcelona Institute of Science and Technology (BIST), lo que convierte a esta institución en líder europea en la última convocatoria del ERC.
De los cuatro investigadores del BIST seleccionados, dos están adscritos al Institut de Recerca Biomèdica (Eduard Batlle y Ángel Nebreda) y otros dos, al Centre de Regulació Genòmica (Miguel Beato y Luis Serrano). Los otros tres investigadores españoles financiados son Albert Quintana, de la UAB; María Vallet-Regí, de la Universidad Complutense; y María J. Vicent, del Centro de Investigación Príncipe Felipe de València.


