Review Articles
Amina Benaicha-Fernández, Stuart P. Atkinson, Inmaculada Conejos-Sánchez, Maria Medel, and María J. Vicent. Polymer-based nanomedicines: Supporting multimodal approaches to glioblastoma multiforme treatment. Advanced Drug Delivery Reviews. 2025. In Press. [ADDR][PubMed]
 Glioblastoma multiforme (GBM) remains one of the most aggressive and lethal cancers affecting the central nervous system (CNS), with significant obstacles precluding effective diagnosis, treatment, and monitoring, including the presence of the blood-brain barrier, tumor heterogeneity, and an immunosuppressive tumor microenvironment. Polymer-based nanomedicines have emerged as a promising approach to overcome these barriers, offering innovative targeted diagnostic and therapeutic strategies for GBM patients. This review now provides an overview of why GBM still represents a diagnostic and therapeutic challenge and then provides a summary of recent high-impact studies that explored how polymers and polypeptides can be employed to promote blood-brain barrier penetration, tumor accumulation, and positive therapeutic outcomes. Additionally, we discuss the use of polymers/polypeptides in the development of multimodal therapies for GBM, including the combination of chemotherapeutic and molecularly targeted drugs/treatments, explore how they can support the combination of distinct therapeutic modalities, such as phototherapy and immunotherapy, in one platform, and describe how they apply to the development of novel GBM theranostic strategies. We also discuss the preclinical validation of polymer-based therapeutic approaches to GBM by exploring recent advances in complex in vitro and in vivo models. Finally, we look forward to the future of GBM treatment with nanomedicines, where we describe emerging therapeutic strategies for GBM and how we may boost the clinical translation of often complex polymer-based nanomedicines. Overall, we believe that this review provides robust evidence for the relevance of polymer-based nanomedicines to the treatment of GBM.
Glioblastoma multiforme (GBM) remains one of the most aggressive and lethal cancers affecting the central nervous system (CNS), with significant obstacles precluding effective diagnosis, treatment, and monitoring, including the presence of the blood-brain barrier, tumor heterogeneity, and an immunosuppressive tumor microenvironment. Polymer-based nanomedicines have emerged as a promising approach to overcome these barriers, offering innovative targeted diagnostic and therapeutic strategies for GBM patients. This review now provides an overview of why GBM still represents a diagnostic and therapeutic challenge and then provides a summary of recent high-impact studies that explored how polymers and polypeptides can be employed to promote blood-brain barrier penetration, tumor accumulation, and positive therapeutic outcomes. Additionally, we discuss the use of polymers/polypeptides in the development of multimodal therapies for GBM, including the combination of chemotherapeutic and molecularly targeted drugs/treatments, explore how they can support the combination of distinct therapeutic modalities, such as phototherapy and immunotherapy, in one platform, and describe how they apply to the development of novel GBM theranostic strategies. We also discuss the preclinical validation of polymer-based therapeutic approaches to GBM by exploring recent advances in complex in vitro and in vivo models. Finally, we look forward to the future of GBM treatment with nanomedicines, where we describe emerging therapeutic strategies for GBM and how we may boost the clinical translation of often complex polymer-based nanomedicines. Overall, we believe that this review provides robust evidence for the relevance of polymer-based nanomedicines to the treatment of GBM.
Camilla Pegoraro, Inés Domingo, Inmaculada Conejos-Sánchez, Maria J. Vicent. Unlocking the Mitochondria for Nanomedicine-based Treatments: Overcoming Biological Barriers, Improving Designs, and Selecting Verification Techniques. Advanced Drug Delivery Reviews. 2024. Apr;207:115195 [ADDR][PubMed]
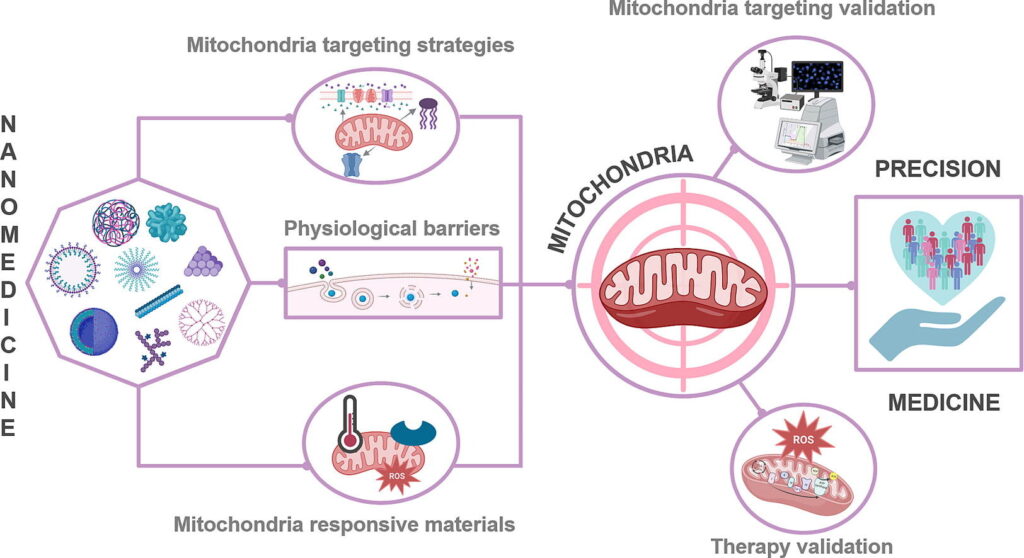 Enhanced targeting approaches will support the treatment of diseases associated with dysfunctional mitochondria, which play critical roles in energy generation and cell survival. Obstacles to mitochondria-specific targeting include the presence of distinct biological barriers and the need to pass through (or avoid) various cell internalization mechanisms. A range of studies have reported the design of mitochondrially-targeted nanomedicines that navigate the complex routes required to influence mitochondrial function; nonetheless, a significant journey lies ahead before mitochondrially-targeted nanomedicines become suitable for clinical use. Moving swiftly forward will require safety studies, in vivo assays confirming effectiveness, and methodologies to validate mitochondria-targeted nanomedicines’ subcellular location/activity. From a nanomedicine standpoint, we describe the biological routes involved (from administration to arrival within the mitochondria), the features influencing rational design, and the techniques used to identify/validate successful targeting. Overall, rationally-designed mitochondria-targeted-based nanomedicines hold great promise for precise subcellular therapeutic delivery.
Enhanced targeting approaches will support the treatment of diseases associated with dysfunctional mitochondria, which play critical roles in energy generation and cell survival. Obstacles to mitochondria-specific targeting include the presence of distinct biological barriers and the need to pass through (or avoid) various cell internalization mechanisms. A range of studies have reported the design of mitochondrially-targeted nanomedicines that navigate the complex routes required to influence mitochondrial function; nonetheless, a significant journey lies ahead before mitochondrially-targeted nanomedicines become suitable for clinical use. Moving swiftly forward will require safety studies, in vivo assays confirming effectiveness, and methodologies to validate mitochondria-targeted nanomedicines’ subcellular location/activity. From a nanomedicine standpoint, we describe the biological routes involved (from administration to arrival within the mitochondria), the features influencing rational design, and the techniques used to identify/validate successful targeting. Overall, rationally-designed mitochondria-targeted-based nanomedicines hold great promise for precise subcellular therapeutic delivery.
Ronit Satchi-Fainaro, Helena F. Florindo, and María J.Vicent (Guest Editors). Editorial: Clinically-relevant and predictive cancer models for nanomedicine evaluation. Advanced Drug Delivery Reviews, 2022; 183: 114140 [ADDR]
The poor clinical translation of nanomedicines has motivated the development of pre-clinical cancer models that better mimic the clinical scenario in each cancer type and stage. To better resemble the clinical setting and considering the paramount role of the immune system in dictating host response to cancer therapy and disease progression, vast efforts have been devoted to evaluating novel cancer therapies in models that allow an integrated analysis of major players within the immune-tumor-stroma complex while ensuring the adequate representation of inter-tumor and intratumor heterogeneity. This Special Issue focused on clinically-relevant and predictive cancer models for nanomedicine evaluation of Advanced Drug Delivery Reviews addresses this challenge by discussing the problems associated with predicting the clinical impact of currently developed immunotherapies and the lack of models that accurately mimic the complex interactions between human tumorigenesis and immune responses within the tumor microenvironment (TME).
Hui Nee Geo, Dharmani Devi Murugan, Zamri Chik, Anwar Norazit, Yiing Yee Foo, Bey Fen Leo, Yin Teo, Sharifah Zamiah Syed Binti Syed Abdul Kadir, Yinghan Chan, Hann Juang Chai, María Medel, Nor Azizan Abdullah, Edward J. Johns, María J. Vicent, Lip Yong Chung, Lik Voon Kiew. Renal Nano-drug Delivery for Acute Kidney Injury: Current Status and Future Perspectives. Journal of Controlled Release, 2022;343, 237-254 [Journal of Controlled Release][PubMed]
 Acute kidney injury (AKI) causes considerable morbidity and mortality, particularly in the case of post-cardiac infarction or kidney transplantation; however, the site-specific accumulation of small molecule reno-protective agents for AKI has often proved ineffective due to dynamic fluid and solute excretion and non-selectivity, which impedes therapeutic efficacy. This article reviews the current status and future trajectories of renal nanomedicine research for AKI management from pharmacological and clinical perspectives, with a particular focus on appraising nanosized drug carrier (NDC) use for the delivery of reno-protective agents of different pharmacological classes and the effectiveness of NDCs in improving renal tissue targeting selectivity and efficacy of said agents. This review reveals the critical shift in the role of the small molecule reno-protective agents in AKI pharmacotherapy – from prophylaxis to treatment – when using NDCs for delivery to the kidney. We also highlight the need to identify the accumulation sites of NDCs carrying reno-protective agents in renal tissues during in vivo assessments and detail the less-explored pharmacological classes of reno-protective agents whose efficacies may be improved via NDC-based delivery. We conclude the paper by outlining the challenges and future perspectives of NDC-based reno-protective agent delivery for better clinical management of AKI.
Acute kidney injury (AKI) causes considerable morbidity and mortality, particularly in the case of post-cardiac infarction or kidney transplantation; however, the site-specific accumulation of small molecule reno-protective agents for AKI has often proved ineffective due to dynamic fluid and solute excretion and non-selectivity, which impedes therapeutic efficacy. This article reviews the current status and future trajectories of renal nanomedicine research for AKI management from pharmacological and clinical perspectives, with a particular focus on appraising nanosized drug carrier (NDC) use for the delivery of reno-protective agents of different pharmacological classes and the effectiveness of NDCs in improving renal tissue targeting selectivity and efficacy of said agents. This review reveals the critical shift in the role of the small molecule reno-protective agents in AKI pharmacotherapy – from prophylaxis to treatment – when using NDCs for delivery to the kidney. We also highlight the need to identify the accumulation sites of NDCs carrying reno-protective agents in renal tissues during in vivo assessments and detail the less-explored pharmacological classes of reno-protective agents whose efficacies may be improved via NDC-based delivery. We conclude the paper by outlining the challenges and future perspectives of NDC-based reno-protective agent delivery for better clinical management of AKI.
Luz Tortajada, Carles Felip*, and María J. Vicent* Polymer-based Non-viral Vectors for Gene Therapy in the Skin. Polymer Chemistry, 2022;13, 718-735 [Polymer Chemistry]
Ramón Martínez Máñez, José Becerra, María Pilar Marco, María Jesús Vicent, Joaquín Arenas, Ángel Carracedo, Pablo Lapunzina, Fernando Martín-Sánchez. Informes Anticipando NANOMEDICINA. Fundación Instituto Roche 2021. ISBN edición online: 978-84-09-34096-5
La nanomedicina es la aplicación de la nanotecnología, es decir, del conocimiento de los eventos ocurridos a escala nanométrica y el desarrollo de nanomateriales, en el campo de la salud. En esta escala, los materiales adquieren propiedades diferentes (ópticas, eléctricas, magnéticas, térmicas, etc.) a sus propiedades macroscópicas, lo que permite, en el ámbito sanitario, el diseño de nuevas aplicaciones con el objetivo de mejorar la calidad de vida de las personas.
Actualmente, existen numerosas aplicaciones basadas en la nanomedicina y su número se encuentra en constante crecimiento, principalmente en el área del nanodiagnóstico y de la nanoterapia, cobrando especial relevancia también el papel de la nanotecnología en la medicina regenerativa. Si bien es cierto que tradicionalmente se han dirigido más esfuerzos al desarrollo de la nanomedicina para combatir el cáncer, a lo largo de este Informe Anticipando se presentan otros ámbitos de aplicación de nanopartículas o nanoestructuras para la liberación controlada de fármacos en otras patologías, nanodispositivos para el diagnóstico de enfermedades o el desarrollo de nanomateriales para aplicaciones en medicina regenerativa.
En los próximos años, y gracias a la traslación a la práctica clínica de cada vez más desarrollos basados en estas tecnologías, la nanomedicina contribuirá a que la medicina del futuro aborde el diagnóstico y tratamien-to de las enfermedades de manera más precoz, más eficaz y de forma personalizada.
Gómez-Cebrián, N., I. Domingo-Ortí, J. L. Poveda, M. J. Vicent, L. Puchades-Carrasco and A. Pineda-Lucena. Multi-Omic Approaches to Breast Cancer Metabolic Phenotyping: Applications in Diagnosis, Prognosis, and the Development of Novel Treatments. Cancers (Basel), 2021;13(18), 4544. [PubMed][Free Download at Cancers]
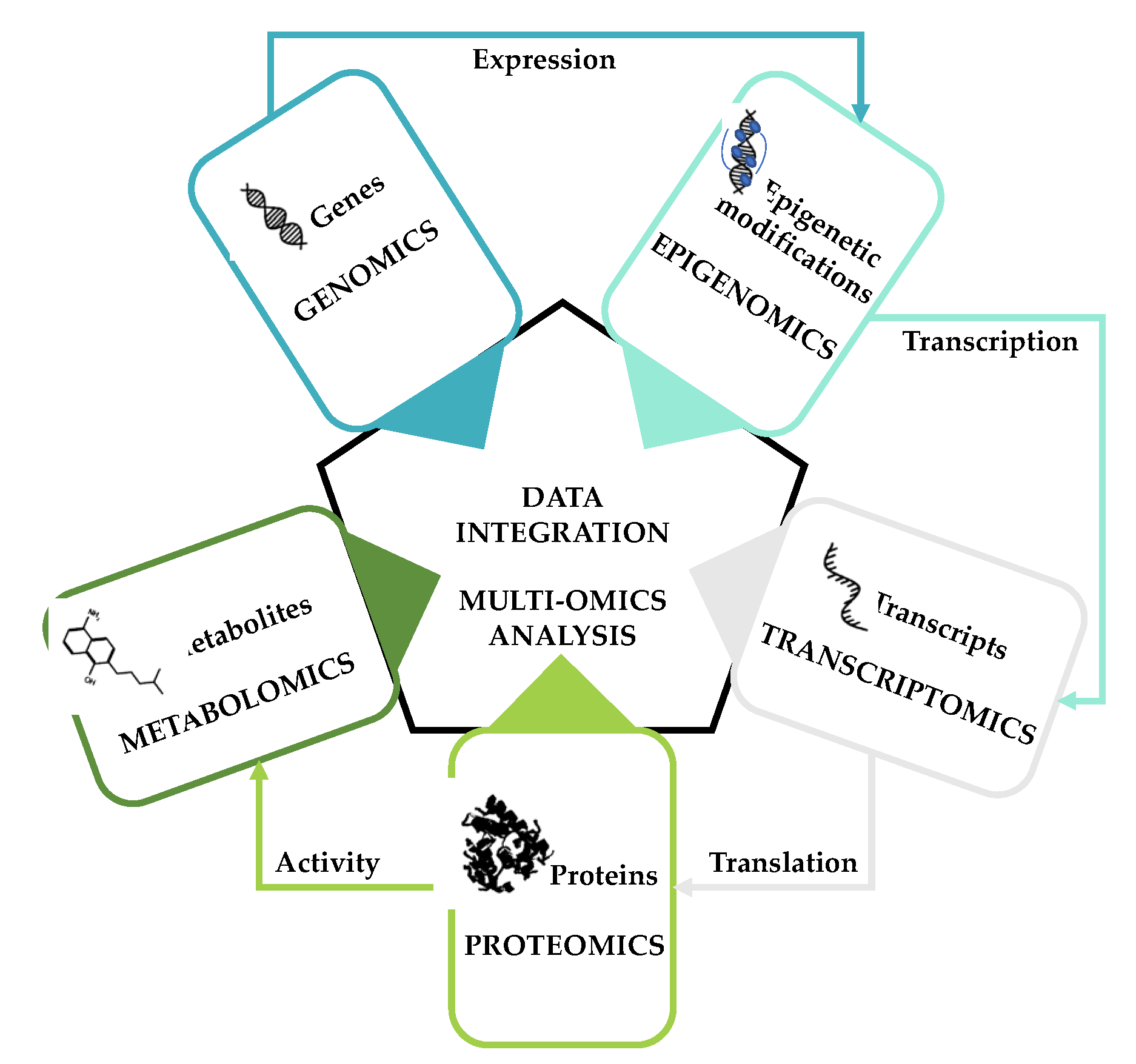 Breast cancer (BC) is characterized by high disease heterogeneity and represents the most frequently diagnosed cancer among women worldwide. Complex and subtype-specific gene expression alterations participate in disease development and progression, with BC cells known to rewire their cellular metabolism to survive, proliferate, and invade. Hence, as an emerging cancer hallmark, metabolic reprogramming holds great promise for cancer diagnosis, prognosis, and treatment. Multi-omics approaches (the combined analysis of various types of omics data) offer opportunities to advance our understanding of the molecular changes underlying metabolic rewiring in complex diseases such as BC. Recent studies focusing on the combined analysis of genomics, epigenomics, transcriptomics, proteomics, and/or metabolomics in different BC subtypes have provided novel insights into the specificities of metabolic rewiring and the vulnerabilities that may guide therapeutic development and improve patient outcomes. This review summarizes the findings of multi-omics studies focused on the characterization of the specific metabolic phenotypes of BC and discusses how they may improve clinical BC diagnosis, subtyping, and treatment.
Breast cancer (BC) is characterized by high disease heterogeneity and represents the most frequently diagnosed cancer among women worldwide. Complex and subtype-specific gene expression alterations participate in disease development and progression, with BC cells known to rewire their cellular metabolism to survive, proliferate, and invade. Hence, as an emerging cancer hallmark, metabolic reprogramming holds great promise for cancer diagnosis, prognosis, and treatment. Multi-omics approaches (the combined analysis of various types of omics data) offer opportunities to advance our understanding of the molecular changes underlying metabolic rewiring in complex diseases such as BC. Recent studies focusing on the combined analysis of genomics, epigenomics, transcriptomics, proteomics, and/or metabolomics in different BC subtypes have provided novel insights into the specificities of metabolic rewiring and the vulnerabilities that may guide therapeutic development and improve patient outcomes. This review summarizes the findings of multi-omics studies focused on the characterization of the specific metabolic phenotypes of BC and discusses how they may improve clinical BC diagnosis, subtyping, and treatment.
Conejos-Sánchez, I., S. Đorđević, M. Medel, and M. J. Vicent*. Polypeptides as Building Blocks for Image-Guided Nanotherapies. Current Opinion in Biomedical Engineering 2021;20:100323. [Free Download at COBE]
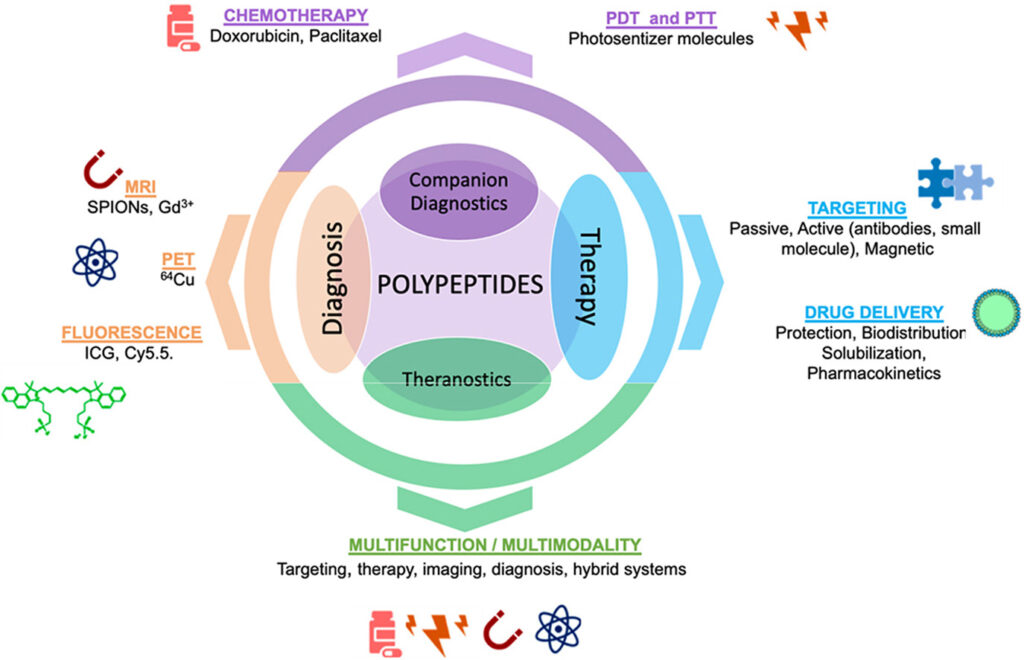 Synthetic polypeptide-based nanomedicines represent highly versatile, advanced therapeutic platforms, with multiple examples currently under clinical evaluation and polypeptidic drugs (Vivagel® and Copaxone™) achieving market-approval. Advances in controlled polymerization techniques, material bioresponsiveness, analytics, and manufacturing in parallel with a deeper biological understanding of pathological sites and biological barriers, have fostered the development of these nature-mimicking entities. Engineered polypeptides could solve challenges currently facing advanced drug delivery, diagnosis, and disease monitoring; however, their contribution to image-guided nanotherapies requires significant development. Herein, we provide critical insight into the current state, challenges, and opportunities of polypeptide-based image-guided nanotherapies, going from companion diagnostics up to nanotheranostic strategies.
Synthetic polypeptide-based nanomedicines represent highly versatile, advanced therapeutic platforms, with multiple examples currently under clinical evaluation and polypeptidic drugs (Vivagel® and Copaxone™) achieving market-approval. Advances in controlled polymerization techniques, material bioresponsiveness, analytics, and manufacturing in parallel with a deeper biological understanding of pathological sites and biological barriers, have fostered the development of these nature-mimicking entities. Engineered polypeptides could solve challenges currently facing advanced drug delivery, diagnosis, and disease monitoring; however, their contribution to image-guided nanotherapies requires significant development. Herein, we provide critical insight into the current state, challenges, and opportunities of polypeptide-based image-guided nanotherapies, going from companion diagnostics up to nanotheranostic strategies.
Đorđević, S., M. M. Gonzalez, I. Conejos-Sánchez, B. Carreira, S. Pozzi, R. C. Acúrcio, R. Satchi-Fainaro*, H. F. Florindo*, and M. J. Vicent*. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Delivery and Translational Research 2021;12:500–525 [PubMed][Journal Website]
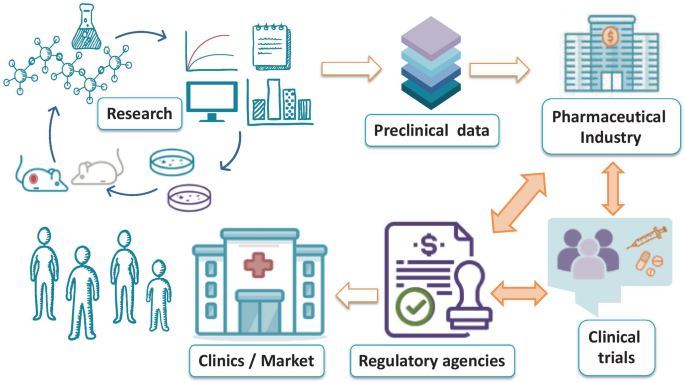
The field of nanomedicine has significantly influenced research areas such as drug delivery, diagnostics, theranostics, and regenerative medicine; however, the further development of this field will face significant challenges at the regulatory level if related guidance remains unclear and unconsolidated. This review describes those features and pathways crucial to the clinical translation of nanomedicine and highlights considerations for early-stage product development. These include identifying those critical quality attributes of the drug product essential for activity and safety, appropriate analytical methods (physical, chemical, biological) for characterization, important process parameters, and adequate pre-clinical models. Additional concerns include the evaluation of batch-to-batch consistency and considerations regarding scaling up that will ensure a successful reproducible manufacturing process. Furthermore, we advise close collaboration with regulatory agencies from the early stages of development to assure an aligned position to accelerate the development of future nanomedicines.
Yin, L., J. Cheng, T. J. Deming and M. J. Vicent. Synthetic Polypeptides for Drug and Gene Delivery, and Tissue Engineering. Advanced Drug Delivery Reviews, 2021:113995. [Journal Website][PubMed].
Peptide and polypeptide materials represent one of the most important classes of biopolymers, which have been widely used in many biomedical applications. Sharing the same backbone with natural proteins, peptides and polypeptides exhibit excellent safety profiles and properties amenable for biomedical applications. While incorporating the twenty-one natural amino acids offers a means to control materials properties, the incorporation of non-natural amino acid residues further endows the materials with chemical diversity. Their ability to spontaneously fold into ordered secondary structures, including α-helices and β-sheets, further enriches the toolbox for researchers to tune the structure, assembly behavior, and biological functions of these materials. The past decade has witnessed significant progress in research on materials design and biomedical applications of peptides and polypeptides. In this themed issue of Advanced Drug Delivery Reviews, leading experts in the field summarize recent advances and accomplishments in peptide and polypeptide materials for biomedical applications. More importantly, they also provide a critical view of emerging directions of the field.
Boix-Montesinos P., Soriano-Teruel P. M., Armiñán A., Orzáez, M, & Vicent M. J. The Past, Present, and Future of Breast Cancer Models for Nanomedicine Development. Advanced Drug Delivery Reviews 2021;173:306-330. [PubMed][Journal Website][Zenodo]
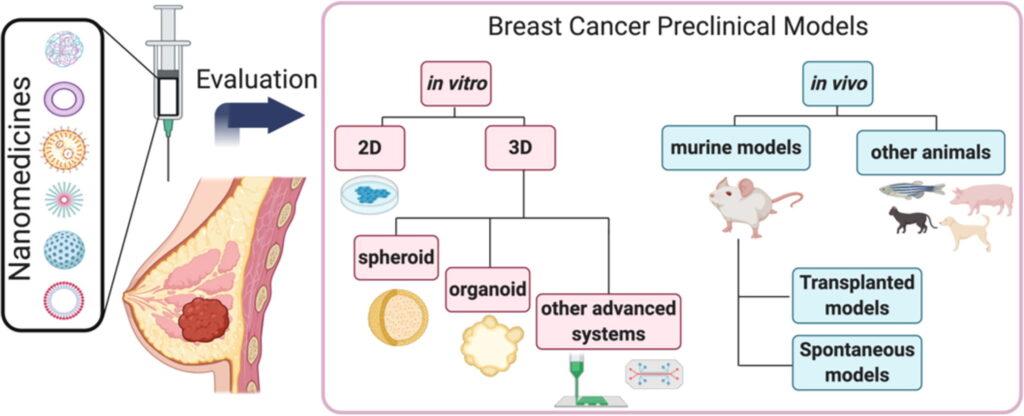 Even given recent advances in nanomedicine development of breast cancer treatment in recent years and promising results in pre-clinical models, cancer nanomedicines often fail at the clinical trial stage. Limitations of conventional in vitro models include the lack of representation of the stromal population, the absence of a three-dimensional (3D) structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. Herein, we review those cell culture strategies that aim to overcome these limitations, including cell co-cultures, advanced 3D cell cultures, patient-derived cells, bioprinting, and microfluidics systems. The in vivo evaluation of nanomedicines must consider critical parameters that include the enhanced permeability and retention effect, the host’s immune status, and the site of tumor implantation. Here, we critically discuss the advantages and limitations of current in vivo models and report how the improved selection and application of breast cancer models can improve the clinical translation of nanomedicines.
Even given recent advances in nanomedicine development of breast cancer treatment in recent years and promising results in pre-clinical models, cancer nanomedicines often fail at the clinical trial stage. Limitations of conventional in vitro models include the lack of representation of the stromal population, the absence of a three-dimensional (3D) structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. Herein, we review those cell culture strategies that aim to overcome these limitations, including cell co-cultures, advanced 3D cell cultures, patient-derived cells, bioprinting, and microfluidics systems. The in vivo evaluation of nanomedicines must consider critical parameters that include the enhanced permeability and retention effect, the host’s immune status, and the site of tumor implantation. Here, we critically discuss the advantages and limitations of current in vivo models and report how the improved selection and application of breast cancer models can improve the clinical translation of nanomedicines.
Silvestri A., Vicente F, Vicent M.J., Stechmann B., Fecke W. Academic collaborative models fostering the translation of physiological in vitro systems from basic research into drug discovery. Drug Discovery Today 2021 Mar 4;S1359-6446(21)00109-4. [Free Download at Drug Discovery Today][PubMed]
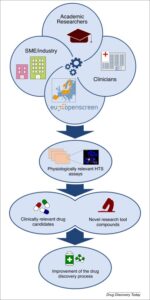 The success of preclinical drug discovery strongly relies on the ability of experimental models to resemble human pathophysiology. The number of compounds receiving approval for clinical use is limited, and this has led to the development of more physiologically relevant cellular models aimed at making preclinical results more prone to be successfully translated into clinical use. In this review, we summarize the technologies available in the field of high-throughput screening (HTS) using complex cellular models, and describe collaborative initiatives, such as EU-OPENSCREEN, which can efficiently support researchers to easily access state-of-the-art chemical biology platforms for improving the drug discovery process.
The success of preclinical drug discovery strongly relies on the ability of experimental models to resemble human pathophysiology. The number of compounds receiving approval for clinical use is limited, and this has led to the development of more physiologically relevant cellular models aimed at making preclinical results more prone to be successfully translated into clinical use. In this review, we summarize the technologies available in the field of high-throughput screening (HTS) using complex cellular models, and describe collaborative initiatives, such as EU-OPENSCREEN, which can efficiently support researchers to easily access state-of-the-art chemical biology platforms for improving the drug discovery process.
Vicente-Ruiz, S., Serrano-Martí, A., Armiñán, A., and Vicent, M. J. Nanomedicine for the Treatment of Advanced Prostate Cancer. Advanced Therapeutics, 2021;4: 2000136. [Journal Website]
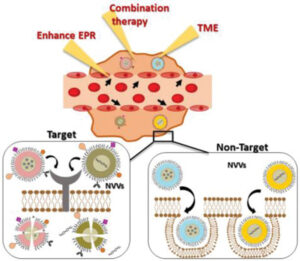 Current prostate cancer (PCa) treatment options include hormonal therapy, chemotherapy, immunotherapy, and radiotherapy; however, a lack of targeting and overall efficiency has failed to improve survival rates or reduce unwanted side‐effects in advanced stage PCa patients. The modification of existing therapeutics, including their reformulation as nanomedicines, can increase stability in plasma, enhance tumor targeting, and improve pharmacokinetics to foster improvements in patient outcomes. This review now describes those nanomedicinal approaches to advanced PCa treatment under evaluation in both preclinical and clinical studies. Despite the current advantages provided by nanomedicine in PCa treatment, there exists the opportunity to improve the design of novel therapeutic approaches. Therefore, this review also highlights the importance of identifying novel functional biomarkers to stratify patients and guide nanomedicinal design. Finally, the authors describe strategies employed to enhance the passive accumulation of nanomedicines in tumors and discuss the enormous potential of combination therapies that target tumor cells and the all‐important tumor microenvironment to reduce tumor growth and overcome therapeutic resistance.
Current prostate cancer (PCa) treatment options include hormonal therapy, chemotherapy, immunotherapy, and radiotherapy; however, a lack of targeting and overall efficiency has failed to improve survival rates or reduce unwanted side‐effects in advanced stage PCa patients. The modification of existing therapeutics, including their reformulation as nanomedicines, can increase stability in plasma, enhance tumor targeting, and improve pharmacokinetics to foster improvements in patient outcomes. This review now describes those nanomedicinal approaches to advanced PCa treatment under evaluation in both preclinical and clinical studies. Despite the current advantages provided by nanomedicine in PCa treatment, there exists the opportunity to improve the design of novel therapeutic approaches. Therefore, this review also highlights the importance of identifying novel functional biomarkers to stratify patients and guide nanomedicinal design. Finally, the authors describe strategies employed to enhance the passive accumulation of nanomedicines in tumors and discuss the enormous potential of combination therapies that target tumor cells and the all‐important tumor microenvironment to reduce tumor growth and overcome therapeutic resistance.
Ghandehari, H., H. K. Chan, H. Harashima, J. A. MacKay, T. Minko, K. Schenke-Layland, Y. Shen, and M. J. Vicent. Advanced Drug Delivery 2020 – Parts 1, 2, and 3. Advanced Drug Delivery Reviews, 2020;156: 1-2. [Journal Website][PubMed]
 Advances in drug delivery are made possible by design and development of novel carriers and delivery methods, better understanding of biological barriers, and by tackling the significant translational challenges for more safe and effective treatment of diseases using these technologies. As the expression “hindsight is 20-20” implies, the editorial team of ADDR felt that in the year 2020 there is an opportunity to articulate what we have learned about advanced drug delivery systems over the years, what are some of the remaining challenges, and what are potential future directions and outcomes. In the next three theme issues of ADDR titled “Advanced Drug Delivery 2020” (Parts I-III), leading experts in the field provide perspectives and reviews on accomplishments in each of these areas and point to challenges and future directions. The collection of articles in these theme issues by no means is meant to be a comprehensive series of articles on important topics in advanced drug delivery, rather the goal is to provide reviews and critical perspectives on selected topics from leading investigators who were able to contribute by providing evidences and their own opinions to offer the Drug Delivery Community with hints on how and where our field is moving to, the challenges to overcome and future opportunities. Since the time of the solicitation of these articles, sadly one of the leading scientists in drug delivery Sung Wan Kim who was invited to contribute suddenly passed away. Dr. Kim was a pioneer in the design and development of polymeric drug and gene delivery systems. To commemorate his significant contributions to the field we dedicate these series to Sung Wan Kim and invite you to read the eulogy written by his long-time colleague Jindřich (Henry) Kopeček.
Advances in drug delivery are made possible by design and development of novel carriers and delivery methods, better understanding of biological barriers, and by tackling the significant translational challenges for more safe and effective treatment of diseases using these technologies. As the expression “hindsight is 20-20” implies, the editorial team of ADDR felt that in the year 2020 there is an opportunity to articulate what we have learned about advanced drug delivery systems over the years, what are some of the remaining challenges, and what are potential future directions and outcomes. In the next three theme issues of ADDR titled “Advanced Drug Delivery 2020” (Parts I-III), leading experts in the field provide perspectives and reviews on accomplishments in each of these areas and point to challenges and future directions. The collection of articles in these theme issues by no means is meant to be a comprehensive series of articles on important topics in advanced drug delivery, rather the goal is to provide reviews and critical perspectives on selected topics from leading investigators who were able to contribute by providing evidences and their own opinions to offer the Drug Delivery Community with hints on how and where our field is moving to, the challenges to overcome and future opportunities. Since the time of the solicitation of these articles, sadly one of the leading scientists in drug delivery Sung Wan Kim who was invited to contribute suddenly passed away. Dr. Kim was a pioneer in the design and development of polymeric drug and gene delivery systems. To commemorate his significant contributions to the field we dedicate these series to Sung Wan Kim and invite you to read the eulogy written by his long-time colleague Jindřich (Henry) Kopeček.
Melnyk, T., Đorđević, S., Conejos-Sánchez, I., and Vicent, M. J. Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation. Advanced Drug Delivery Reviews 2020; 160: 136-169. [Journal Website][Zenodo][PubMed]
 The clinical success of polypeptides as polymeric drugs, covered by the umbrella term “polymer therapeutics,” combined with related scientific and technological breakthroughs, explain their exponential growth in the development of polypeptide-drug conjugates as therapeutic agents. A deeper understanding of the biology at relevant pathological sites and the critical biological barriers faced, combined with advances regarding controlled polymerization techniques, material bioresponsiveness, analytical methods, and scale up-manufacture processes, have fostered the development of these nature-mimicking entities. Now, engineered polypeptides have the potential to combat current challenges in the advanced drug delivery field. In this review, we will discuss examples of polypeptide-drug conjugates as single or combination therapies in both preclinical and clinical studies as therapeutics and molecular imaging tools. Importantly, we will critically discuss relevant examples to highlight those parameters relevant to their rational design, such as linking chemistry, the analytical strategies employed, and their physicochemical and biological characterization, that will foster their rapid clinical translation.
The clinical success of polypeptides as polymeric drugs, covered by the umbrella term “polymer therapeutics,” combined with related scientific and technological breakthroughs, explain their exponential growth in the development of polypeptide-drug conjugates as therapeutic agents. A deeper understanding of the biology at relevant pathological sites and the critical biological barriers faced, combined with advances regarding controlled polymerization techniques, material bioresponsiveness, analytical methods, and scale up-manufacture processes, have fostered the development of these nature-mimicking entities. Now, engineered polypeptides have the potential to combat current challenges in the advanced drug delivery field. In this review, we will discuss examples of polypeptide-drug conjugates as single or combination therapies in both preclinical and clinical studies as therapeutics and molecular imaging tools. Importantly, we will critically discuss relevant examples to highlight those parameters relevant to their rational design, such as linking chemistry, the analytical strategies employed, and their physicochemical and biological characterization, that will foster their rapid clinical translation.
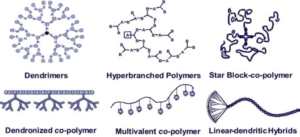
Moura, LIF., Malfanti, A., Peres, C., Matos, AI., Guegain, E., Sainz, W., Zloh, Vicent, MJ., Florindo, HF. Functionalized Branched Polymers: Promising Immunomodulatory Tools for the Treatment of Cancer and Immune Disorders. Materials Horizons, 2019;6:1956-1973 [Journal Website][Zenodo]
 Well-defined synthetic branched nanostructures form an emerging subclass of macromolecular structures, whose 3D structure and multivalency offer unique opportunities for fine-tuning their internalization and cellular targeting. In particular, dendrimers possess a well-defined 3D-globular backbone with highly versatile functional surface groups and exhibit a range of chemical and biological properties. Branched polymers present unique opportunities for the targeted delivery of diverse bioactive molecules (including targeting ligands, imaging probes, and drugs) via conjugation to multiple sites within the structure. The inherent versatility and multifunctionality of these architectures make them potentially useful for the modulation of multiple immune-related pathways for the treatment of a wide range of disease and disorders, including cancer and human immunodeficiency virus infection. Herein, we describe the key components of the immune system whose targeting can help to overcome immune-related disorders and discuss branched polymers (including dendrimers) as promising delivery systems with unique immunomodulatory properties against cancer and infectious diseases.
Well-defined synthetic branched nanostructures form an emerging subclass of macromolecular structures, whose 3D structure and multivalency offer unique opportunities for fine-tuning their internalization and cellular targeting. In particular, dendrimers possess a well-defined 3D-globular backbone with highly versatile functional surface groups and exhibit a range of chemical and biological properties. Branched polymers present unique opportunities for the targeted delivery of diverse bioactive molecules (including targeting ligands, imaging probes, and drugs) via conjugation to multiple sites within the structure. The inherent versatility and multifunctionality of these architectures make them potentially useful for the modulation of multiple immune-related pathways for the treatment of a wide range of disease and disorders, including cancer and human immunodeficiency virus infection. Herein, we describe the key components of the immune system whose targeting can help to overcome immune-related disorders and discuss branched polymers (including dendrimers) as promising delivery systems with unique immunomodulatory properties against cancer and infectious diseases.
Brennecke P, Rasina D, Aubi O, Herzog K, Landskron J, Cautain B, Vicente F, Quintana J, Mestres J, Stechmann B, Ellinger B, Brea J, Kolanowski JL, Pilarski R, Orzaez M, Pineda-Lucena A, Laraia L, Nami F, Zielenkiewicz P, Paruch K, Hansen E, von Kries JP, Neuenschwander M, Specker E, Bartunek P, Simova S, Leśnikowski Z, Krauss S, Lehtiö L, Bilitewski U, Brönstrup M, Taskén K, Jirgensons A, Lickert H, Clausen MH, Andersen JH, Vicent MJ, Genilloud O, Martinez A, Nazaré M, Fecke W, Gribbon P. EU-OPENSCREEN: A Novel Collaborative Approach to Facilitate Chemical Biology. SLAS Discovery, 2019;7:2472555218816276. [PubMed][SLAS Discovery]
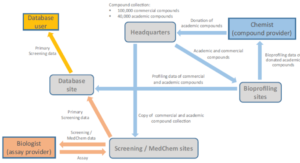 Compound screening in biological assays and subsequent optimization of hits is indispensable for the development of new molecular research tools and drug candidates. To facilitate such discoveries, the European Research Infrastructure EU-OPENSCREEN was founded recently with the support of its member countries and the European Commission. Its distributed character harnesses complementary knowledge, expertise, and instrumentation in the discipline of chemical biology from 20 European partners, and its open working model ensures that academia and industry can readily access EU-OPENSCREEN’s compound collection, equipment, and generated data. To demonstrate the power of this collaborative approach, this perspective article highlights recent projects from EU-OPENSCREEN partner institutions. These studies yielded (1) 2-aminoquinazolin-4(3 H)-ones as potential lead structures for new antimalarial drugs, (2) a novel lipodepsipeptide specifically inducing apoptosis in cells deficient for the pVHL tumor suppressor, (3) small-molecule-based ROCK inhibitors that induce definitive endoderm formation and can potentially be used for regenerative medicine, (4) potential pharmacological chaperones for inborn errors of metabolism and a familiar form of acute myeloid leukemia (AML), and (5) novel tankyrase inhibitors that entered a lead-to-candidate program. Collectively, these findings highlight the benefits of small-molecule screening, the plethora of assay designs, and the close connection between screening and medicinal chemistry within EU-OPENSCREEN.
Compound screening in biological assays and subsequent optimization of hits is indispensable for the development of new molecular research tools and drug candidates. To facilitate such discoveries, the European Research Infrastructure EU-OPENSCREEN was founded recently with the support of its member countries and the European Commission. Its distributed character harnesses complementary knowledge, expertise, and instrumentation in the discipline of chemical biology from 20 European partners, and its open working model ensures that academia and industry can readily access EU-OPENSCREEN’s compound collection, equipment, and generated data. To demonstrate the power of this collaborative approach, this perspective article highlights recent projects from EU-OPENSCREEN partner institutions. These studies yielded (1) 2-aminoquinazolin-4(3 H)-ones as potential lead structures for new antimalarial drugs, (2) a novel lipodepsipeptide specifically inducing apoptosis in cells deficient for the pVHL tumor suppressor, (3) small-molecule-based ROCK inhibitors that induce definitive endoderm formation and can potentially be used for regenerative medicine, (4) potential pharmacological chaperones for inborn errors of metabolism and a familiar form of acute myeloid leukemia (AML), and (5) novel tankyrase inhibitors that entered a lead-to-candidate program. Collectively, these findings highlight the benefits of small-molecule screening, the plethora of assay designs, and the close connection between screening and medicinal chemistry within EU-OPENSCREEN.
Rodriguez‐Otormin, F., Duro‐Castaño, A., Conejos‐Sánchez, I., Vicent, M.J. Envisioning the Future of Polymer Therapeutics for Brain Disorders. WIREs Nanomedicine and Nanobiotechnology, 2019;11:e1532. [PubMed][Journal Website][Zenodo][Advanced Science News][Inside Cover Image]
 The growing incidence of brain‐related pathologies and the problems that undermine the development of efficient and effective treatments have prompted both researchers and the pharmaceutical industry to search for novel therapeutic alternatives. Polymer therapeutics (PT) display properties well suited to the treatment of neuro‐related disorders, which help to overcome the many hidden obstacles on the journey to the central nervous system (CNS). The inherent features of PT, derived from drug(s) conjugation, in parallel with the progress in synthesis and analytical methods, the increasing knowledge in molecular basis of diseases, and collected clinical data through the last four decades, have driven the translation from “bench to bedside” for various biomedical applications. However, since the approval of Gliadel® wafers, little progress has been made in the CNS field, even though brain targeting represents an ever‐growing challenge. A thorough assessment of the steps required for successful brain delivery via different administration routes and the consideration of the disease‐specific hallmarks are essential to progress in the field. Within this review, we hope to summarize the latest developments, successes, and failures and discuss considerations on designs and strategies for PT in the treatment of CNS disorders.
The growing incidence of brain‐related pathologies and the problems that undermine the development of efficient and effective treatments have prompted both researchers and the pharmaceutical industry to search for novel therapeutic alternatives. Polymer therapeutics (PT) display properties well suited to the treatment of neuro‐related disorders, which help to overcome the many hidden obstacles on the journey to the central nervous system (CNS). The inherent features of PT, derived from drug(s) conjugation, in parallel with the progress in synthesis and analytical methods, the increasing knowledge in molecular basis of diseases, and collected clinical data through the last four decades, have driven the translation from “bench to bedside” for various biomedical applications. However, since the approval of Gliadel® wafers, little progress has been made in the CNS field, even though brain targeting represents an ever‐growing challenge. A thorough assessment of the steps required for successful brain delivery via different administration routes and the consideration of the disease‐specific hallmarks are essential to progress in the field. Within this review, we hope to summarize the latest developments, successes, and failures and discuss considerations on designs and strategies for PT in the treatment of CNS disorders.
Atkinson, S.P., Andreu, Z., and Vicent, M.J., Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. Journal of Personalized Medicine, 2018. 8(1): p. 6. [PubMed][Free Download at JPM][Zenodo]
 Polymer therapeutics (PTs) provides a potentially exciting approach for the treatment of many diseases by enhancing aqueous solubility and altering drug pharmacokinetics at both the whole organism and subcellular level leading to improved therapeutic outcomes. However, the failure of many polymer-drug conjugates in clinical trials suggests that we may need to stratify patients in order to match each patient to the right PT. In this concise review, we hope to assess potential PT-specific biomarkers for cancer treatment, with a focus on new studies, detection methods, new models and the opportunities this knowledge will bring for the development of novel PT-based anti-cancer strategies. We discuss the various “hurdles” that a given PT faces on its passage from the syringe to the tumor (and beyond), including the passage through the bloodstream, tumor targeting, tumor uptake and the intracellular release of the active agent. However, we also discuss other relevant concepts and new considerations in the field, which we hope will provide new insight into the possible applications of PT-related biomarkers.
Polymer therapeutics (PTs) provides a potentially exciting approach for the treatment of many diseases by enhancing aqueous solubility and altering drug pharmacokinetics at both the whole organism and subcellular level leading to improved therapeutic outcomes. However, the failure of many polymer-drug conjugates in clinical trials suggests that we may need to stratify patients in order to match each patient to the right PT. In this concise review, we hope to assess potential PT-specific biomarkers for cancer treatment, with a focus on new studies, detection methods, new models and the opportunities this knowledge will bring for the development of novel PT-based anti-cancer strategies. We discuss the various “hurdles” that a given PT faces on its passage from the syringe to the tumor (and beyond), including the passage through the bloodstream, tumor targeting, tumor uptake and the intracellular release of the active agent. However, we also discuss other relevant concepts and new considerations in the field, which we hope will provide new insight into the possible applications of PT-related biomarkers.Editorial: This special issue of the Journal of Drug Targeting marks the past, and the continuing contribution of Ruth Duncan to the field of drug delivery, more specifically the branch termed “Polymer Therapeutics”. The three of us have witnessed, for more than 20 years, Ruth’s never-ending enthusiasm and persistence in many different interdisciplinary arenas under this banner, and the immense scientific contribution to the field of drug delivery this vision (and tenacity) has generated. It has been a pleasure for us to edit this special issue, which pays tribute to her outstanding achievements within the context of the Journal of Drug Targeting- Lifetime Achievement Award for 2017.
Duro-Castano, A., Gallon, E., Decker, C., and Vicent, M.J.*, Modulating angiogenesis with integrin-targeted nanomedicines. Advanced Drug Delivery Reviews, 2017. 119(Supplement C): p. 101-119.[PubMed] [Zenodo]
 Targeting angiogenesis-related pathologies, which include tumorigenesis and metastatic processes, has become an attractive strategy for the development of efficient guided nanomedicines. In this respect, integrins are cell-adhesion molecules involved in angiogenesis signaling pathways and are overexpressed in many angiogenic processes. Therefore, they represent specific biomarkers not only to monitor disease progression but also to rationally design targeted nanomedicines. Arginine-glycine-aspartic (RGD) containing peptides that bind to specific integrins have been widely utilized to provide ligand-mediated targeting capabilities to small molecules, peptides, proteins, and antibodies, as well as to drug/imaging agent-containing nanomedicines, with the final aim of maximizing their therapeutic index. Within this review, we aim to cover recent and relevant examples of different integrin-assisted nanosystems including polymeric nanoconstructs, liposomes, and inorganic nanoparticles applied in drug/gene therapy as well as imaging and theranostics. We will also critically address the overall benefits of integrin-targeting.
Targeting angiogenesis-related pathologies, which include tumorigenesis and metastatic processes, has become an attractive strategy for the development of efficient guided nanomedicines. In this respect, integrins are cell-adhesion molecules involved in angiogenesis signaling pathways and are overexpressed in many angiogenic processes. Therefore, they represent specific biomarkers not only to monitor disease progression but also to rationally design targeted nanomedicines. Arginine-glycine-aspartic (RGD) containing peptides that bind to specific integrins have been widely utilized to provide ligand-mediated targeting capabilities to small molecules, peptides, proteins, and antibodies, as well as to drug/imaging agent-containing nanomedicines, with the final aim of maximizing their therapeutic index. Within this review, we aim to cover recent and relevant examples of different integrin-assisted nanosystems including polymeric nanoconstructs, liposomes, and inorganic nanoparticles applied in drug/gene therapy as well as imaging and theranostics. We will also critically address the overall benefits of integrin-targeting.
Cheah, H.Y., Kiew, L.V., Lee, H.B., Japundžić-Žigon, N., Vicent, M.J., Hoe, S.Z., and Chung, L.Y., Preclinical safety assessments of nano-sized constructs on cardiovascular system toxicity: A case for telemetry. Journal of Applied Toxicology, 2017. 37(11): p. 1268-1285. [PubMed]
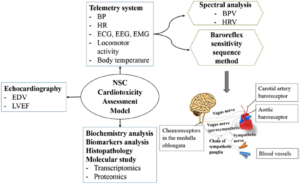 While nano-sized construct (NSC) use in medicine has grown significantly in recent years, reported unwanted side effects have raised safety concerns. However, the toxicity of NSCs to the cardiovascular system (CVS) and the relative merits of the associated evaluation methods have not been thoroughly studied. This review discusses the toxicological profiles of selected NSCs and provides an overview of the assessment methods, including in silico, in vitro, ex vivo and in vivo models and how they are related to CVS toxicity. We conclude the review by outlining the merits of telemetry coupled with spectral analysis, baroreceptor reflex sensitivity analysis and echocardiography as an appropriate integrated strategy for the assessment of the acute and chronic impact of NSCs on the CVS.
While nano-sized construct (NSC) use in medicine has grown significantly in recent years, reported unwanted side effects have raised safety concerns. However, the toxicity of NSCs to the cardiovascular system (CVS) and the relative merits of the associated evaluation methods have not been thoroughly studied. This review discusses the toxicological profiles of selected NSCs and provides an overview of the assessment methods, including in silico, in vitro, ex vivo and in vivo models and how they are related to CVS toxicity. We conclude the review by outlining the merits of telemetry coupled with spectral analysis, baroreceptor reflex sensitivity analysis and echocardiography as an appropriate integrated strategy for the assessment of the acute and chronic impact of NSCs on the CVS.
Zagorodko, O., Arroyo-Crespo, J.J., Nebot, V.J., and Vicent, M.J.*, Polypeptide-Based Conjugates as Therapeutics: Opportunities and Challenges. Macromol Biosci, 2017. 17(1). [PubMed] [Zenodo]
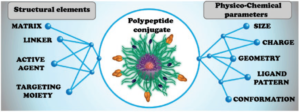 Synthetic polypeptides or polyamino acids have become a useful and multifunctional platform in advanced drug delivery studies. Nonetheless, the full potential of these systems has yet to be achieved. The final structure of polypeptide conjugates and their in vivo behavior are dependent on an extraordinarily complex pattern of interconnected physico-chemical and structural parameters, making sophisticated directional design of such systems difficult and often unachievable. In this review, the authors aim to discuss the role of these parameters in the successful design of different drug delivery architectures and to delineate some basic correlations between structure, properties, and the biological behavior of polypeptide-based conjugates.
Synthetic polypeptides or polyamino acids have become a useful and multifunctional platform in advanced drug delivery studies. Nonetheless, the full potential of these systems has yet to be achieved. The final structure of polypeptide conjugates and their in vivo behavior are dependent on an extraordinarily complex pattern of interconnected physico-chemical and structural parameters, making sophisticated directional design of such systems difficult and often unachievable. In this review, the authors aim to discuss the role of these parameters in the successful design of different drug delivery architectures and to delineate some basic correlations between structure, properties, and the biological behavior of polypeptide-based conjugates.
This review article was one of the journal’s top 20 most downloaded recent papers among articles published between July 2016 and June 2018.
Nino-Pariente, A., Nebot, V.J., and Vicent, M.J.*, Relevant Physicochemical Descriptors of “Soft Nanomedicines” to Bypass Biological Barriers. Curr Pharm Des, 2016. 22(9): p. 1274-91. [PubMed] [Zenodo]
 Herein, we present an overview on the current status of the characterization techniques and methodologies used to study the physicochemical descriptors that influence the final clinical performance of a given nanomedicine. The described techniques were selected based on their suitability to operate under relevant “native” conditions that mimic the physiological environment. Special emphasis is placed on those techniques that hold a greater potential to unravel dynamic, structural, and compositional features of soft organic nanomedicines relevant to the ability to bypass biological barriers, and hence allow for the rational design of drug delivery platforms with improved biological output.
Herein, we present an overview on the current status of the characterization techniques and methodologies used to study the physicochemical descriptors that influence the final clinical performance of a given nanomedicine. The described techniques were selected based on their suitability to operate under relevant “native” conditions that mimic the physiological environment. Special emphasis is placed on those techniques that hold a greater potential to unravel dynamic, structural, and compositional features of soft organic nanomedicines relevant to the ability to bypass biological barriers, and hence allow for the rational design of drug delivery platforms with improved biological output.
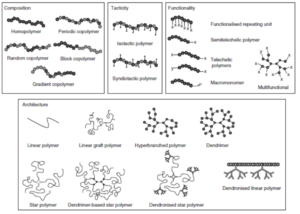
Duro-Castano, A., Movellan, J., and Vicent, M.J.*, Smart branched polymer drug conjugates as nano-sized drug delivery systems. Biomater Sci, 2015. 3(10): p. 1321-34. [PubMed]
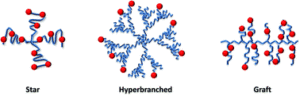 Polymer-drug conjugates represent excellent nanopharmaceutical candidates, as they offer multiple advantages related to their intrinsic characteristics. Many of the said characteristics are provided by the covalent bonding between the drug and the polymer. However, their clinical development has been slow and only one polymer-drug conjugate has reached the market, thus there remains an urgent need for the development of new and smart polymeric systems. Desirable characteristics of these new systems include higher molecular weight and degree of homogeneity, predictable conformations in solution, multivalency, and increased drug loading capacity, amongst others. With these aims in mind, branched polymers are ideal candidates due to their unique rheological, mechanical, and biomedical properties derived from their structure, inaccessible for linear polymers. Within this review, the synthetic strategies developed and the main efforts towards branched polymer implementation as carriers for polymer-drug conjugates will be addressed.
Polymer-drug conjugates represent excellent nanopharmaceutical candidates, as they offer multiple advantages related to their intrinsic characteristics. Many of the said characteristics are provided by the covalent bonding between the drug and the polymer. However, their clinical development has been slow and only one polymer-drug conjugate has reached the market, thus there remains an urgent need for the development of new and smart polymeric systems. Desirable characteristics of these new systems include higher molecular weight and degree of homogeneity, predictable conformations in solution, multivalency, and increased drug loading capacity, amongst others. With these aims in mind, branched polymers are ideal candidates due to their unique rheological, mechanical, and biomedical properties derived from their structure, inaccessible for linear polymers. Within this review, the synthetic strategies developed and the main efforts towards branched polymer implementation as carriers for polymer-drug conjugates will be addressed.
Duro-Castano, A., Conejos-Sánchez, I., and Vicent, M.J.*, Peptide-Based Polymer Therapeutics. Polymers, 2014. 6(2): p. 515. [Link]
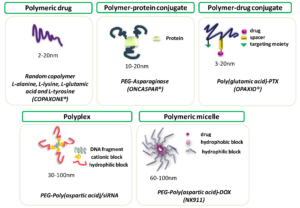 Polypeptides are envisaged to achieve a major impact on a number of different relevant areas such as biomedicine and biotechnology. Acquired knowledge and the increasing interest on amino acids, peptides and proteins is establishing a large panel of these biopolymers whose physical, chemical and biological properties are ruled by their controlled sequences and composition. Polymer therapeutics has helped to establish these polypeptide-based constructs as polymeric nanomedicines for different applications, such as disease treatment and diagnostics. Herein, we provide an overview of the advantages of these systems and the main methodologies for their synthesis, highlighting the different polypeptide architectures and the current research towards clinical applications.
Polypeptides are envisaged to achieve a major impact on a number of different relevant areas such as biomedicine and biotechnology. Acquired knowledge and the increasing interest on amino acids, peptides and proteins is establishing a large panel of these biopolymers whose physical, chemical and biological properties are ruled by their controlled sequences and composition. Polymer therapeutics has helped to establish these polypeptide-based constructs as polymeric nanomedicines for different applications, such as disease treatment and diagnostics. Herein, we provide an overview of the advantages of these systems and the main methodologies for their synthesis, highlighting the different polypeptide architectures and the current research towards clinical applications.
Duncan, R. and Vicent, M.J.*, Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev, 2013. 65(1): p. 60-70. [PubMed]
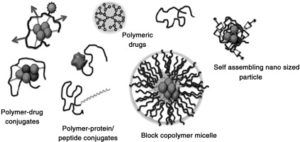 The term “polymer therapeutics” was coined to describe polymeric drugs, polymer conjugates of proteins, drugs and aptamers, together with those block copolymer micelles and multicomponent non-viral vectors which contain covalent linkages. These often complex, multicomponent constructs are actually “drugs” and “macromolecular prodrugs” in contrast to drug delivery systems that simply entrap (non-covalently) therapeutic agents. They have also been described as nanomedicines. First polymer-protein conjugates entered routine clinical use in 1990 and a growing number of polymeric drugs/sequestrants and PEGylated proteins/aptamers have since come into the market. Valuable lessons have been learnt over >3 decades of clinical development, especially in relation to critical product attributes governing safety and efficacy, the validated methods needed for product characterisation. Not least there has been improved understanding of polymer therapeutic-specific biomarkers that will in future enable improved selection of patients for therapy. Advances in synthetic polymer chemistry (including control of 3D architecture), the move towards greater use of biodegradable polymers, polymers delivering combination therapy, increased understanding of polymer therapeutic critical product attributes to guide pharmaceutical development, and advances in understanding of endocytosis and intracellular trafficking pathways in health and disease are opening new opportunities for design and clinical use of polymer-based therapeutics in the decades to come.
The term “polymer therapeutics” was coined to describe polymeric drugs, polymer conjugates of proteins, drugs and aptamers, together with those block copolymer micelles and multicomponent non-viral vectors which contain covalent linkages. These often complex, multicomponent constructs are actually “drugs” and “macromolecular prodrugs” in contrast to drug delivery systems that simply entrap (non-covalently) therapeutic agents. They have also been described as nanomedicines. First polymer-protein conjugates entered routine clinical use in 1990 and a growing number of polymeric drugs/sequestrants and PEGylated proteins/aptamers have since come into the market. Valuable lessons have been learnt over >3 decades of clinical development, especially in relation to critical product attributes governing safety and efficacy, the validated methods needed for product characterisation. Not least there has been improved understanding of polymer therapeutic-specific biomarkers that will in future enable improved selection of patients for therapy. Advances in synthetic polymer chemistry (including control of 3D architecture), the move towards greater use of biodegradable polymers, polymers delivering combination therapy, increased understanding of polymer therapeutic critical product attributes to guide pharmaceutical development, and advances in understanding of endocytosis and intracellular trafficking pathways in health and disease are opening new opportunities for design and clinical use of polymer-based therapeutics in the decades to come.
Perez-Paya, E., Orzaez, M., Mondragon, L., Wolan, D., Wells, J.A., Messeguer, A., and Vicent, M.J.*, Molecules that modulate Apaf-1 activity. Med Res Rev, 2011. 31(4): p. 649-75. [PubMed]
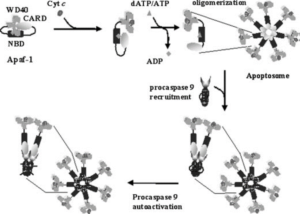 Programmed cell death, apoptosis, is a highly regulated cellular pathway, responsible for the elimination of cells in the organism that are no longer needed or extensively damaged. Defects in the regulation of apoptosis could be at the molecular basis of different diseases, either when it is insufficient or excessive. The formation of the macromolecular complex, apoptosome, is a key event in this pathway, which has also been defined as the intrinsic apoptosis pathway. The apoptosome is a holoenzyme multiprotein complex formed by cytochrome c-activated apoptotic protease-activating factor (Apaf-1), dATP, and procaspase-9. Recent studies have produced a wealth of information about the regulation and functions of Apaf-1, but additional studies aimed at elucidating its role as a signaling device at the crosstalk between different signaling pathways are needed to take advantage for the development of modulators of apoptosis pathways and possible therapeutic applications.
Programmed cell death, apoptosis, is a highly regulated cellular pathway, responsible for the elimination of cells in the organism that are no longer needed or extensively damaged. Defects in the regulation of apoptosis could be at the molecular basis of different diseases, either when it is insufficient or excessive. The formation of the macromolecular complex, apoptosome, is a key event in this pathway, which has also been defined as the intrinsic apoptosis pathway. The apoptosome is a holoenzyme multiprotein complex formed by cytochrome c-activated apoptotic protease-activating factor (Apaf-1), dATP, and procaspase-9. Recent studies have produced a wealth of information about the regulation and functions of Apaf-1, but additional studies aimed at elucidating its role as a signaling device at the crosstalk between different signaling pathways are needed to take advantage for the development of modulators of apoptosis pathways and possible therapeutic applications.
Barz, M., Luxenhofer, R., Zentel, R., and Vicent, M.J.*, Overcoming the PEG-addiction: well-defined alternatives to PEG, from structure-property relationships to better defined therapeutics. Polymer Chemistry, 2011. 2(9): p. 1900-1918. [Link]
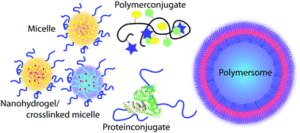 Synthetic methods in polymer chemistry have evolved tremendously during the last decade. Nowadays more and more attention is devoted to the application of those tools in the development of the next generation of nanomedicines. Nevertheless, poly(ethylene glycol) (PEG) remains the most frequently used polymer for biomedical applications. In this review, we try to summarize recent efforts and developments in controlled polymerisation techniques that may allow alternatives to PEG based systems and can be used to improve the properties of future polymer therapeutics. [Journal Website]
Synthetic methods in polymer chemistry have evolved tremendously during the last decade. Nowadays more and more attention is devoted to the application of those tools in the development of the next generation of nanomedicines. Nevertheless, poly(ethylene glycol) (PEG) remains the most frequently used polymer for biomedical applications. In this review, we try to summarize recent efforts and developments in controlled polymerisation techniques that may allow alternatives to PEG based systems and can be used to improve the properties of future polymer therapeutics. [Journal Website]
Canal, F., Sanchis, J., and Vicent, M.J.*, Polymer–drug conjugates as nano-sized medicines. Curr Opin Biotechnol, 2011. 22(6): p. 894-900. [PubMed]
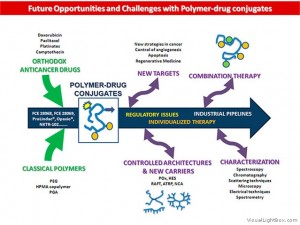 Polymer Therapeutics have enormously evolved in the past decades. Several polymeric drugs as well as polymer-protein conjugates have been in the market since the 90s, but although polymer-drug conjugates are already in clinical trials they still need to reach this final goal. There are four main convergent strategies to move this platform technology further. First, exploitation of new molecular targets in cancer therapy and design of polymer-drug conjugates as treatments for other diseases. Second, the development of combination therapy. Third, attempts to improve polymer chemistry, including the use of new well-defined architectures and the optimization of the advanced characterization techniques essential to transform a promising conjugate into a candidate for clinical evaluation. Finally, increased understanding of polymer conjugate features that govern clinical risk-benefit is leading to an appreciation of clinical biomarkers that will open new possibilities for personalized therapy.
Polymer Therapeutics have enormously evolved in the past decades. Several polymeric drugs as well as polymer-protein conjugates have been in the market since the 90s, but although polymer-drug conjugates are already in clinical trials they still need to reach this final goal. There are four main convergent strategies to move this platform technology further. First, exploitation of new molecular targets in cancer therapy and design of polymer-drug conjugates as treatments for other diseases. Second, the development of combination therapy. Third, attempts to improve polymer chemistry, including the use of new well-defined architectures and the optimization of the advanced characterization techniques essential to transform a promising conjugate into a candidate for clinical evaluation. Finally, increased understanding of polymer conjugate features that govern clinical risk-benefit is leading to an appreciation of clinical biomarkers that will open new possibilities for personalized therapy.
Sanchis, J., Canal, F., Lucas, R., and Vicent, M.J.*, Polymer-drug conjugates for novel molecular targets. Nanomedicine (Lond), 2010. 5(6): p. 915-35. [PubMed]
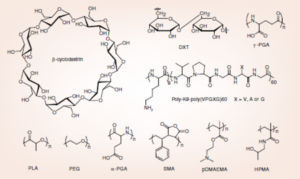 Polymer therapeutics can be already considered as a promising field in the human healthcare context. The discovery of the enhanced permeability and retention effect by Maeda, together with the modular model for the polymer-drug conjugate proposed by Ringsdorf, directed the early steps of polymer therapeutics towards cancer therapy. Orthodox anticancer drugs were preferentially chosen in the development of the first conjugates. The fast evolution of polymer chemistry and bioconjugation techniques, and a deeper understanding of cell biology has opened up exciting new challenges and opportunities. Four main directions have to be considered to develop this ‘platform technology’ further: the control of the synthetic process, the exhaustive characterization of the conjugate architectures, the conquest of combination therapy and the disclosure of new therapeutic targets. We illustrate in this article the exciting approaches offered by polymer-drug conjugates beyond classical cancer therapy, focusing on new, more effective and selective targets in cancer and in their use as treatments for other major human diseases.
Polymer therapeutics can be already considered as a promising field in the human healthcare context. The discovery of the enhanced permeability and retention effect by Maeda, together with the modular model for the polymer-drug conjugate proposed by Ringsdorf, directed the early steps of polymer therapeutics towards cancer therapy. Orthodox anticancer drugs were preferentially chosen in the development of the first conjugates. The fast evolution of polymer chemistry and bioconjugation techniques, and a deeper understanding of cell biology has opened up exciting new challenges and opportunities. Four main directions have to be considered to develop this ‘platform technology’ further: the control of the synthetic process, the exhaustive characterization of the conjugate architectures, the conquest of combination therapy and the disclosure of new therapeutic targets. We illustrate in this article the exciting approaches offered by polymer-drug conjugates beyond classical cancer therapy, focusing on new, more effective and selective targets in cancer and in their use as treatments for other major human diseases.
Duncan, R. and Vicent, M.J.*, Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv Drug Deliv Rev, 2010. 62(2): p. 272-82. [PubMed]
 N-(2-Hydroxypropyl)methacrylamide (HPMA) copolymer conjugates containing doxorubicin designed in the late 1970s/early 1980s as anticancer polymer therapeutics were the first synthetic polymer-based anticancer conjugates to enter clinical trial beginning in 1994. Early clinical results were promising, confirming activity in chemotherapy refractory patients and the safety of HPMA copolymers as a new polymer platform in this setting. Subsequent Phase I/II trials have investigated conjugates containing paclitaxel (PNU 166945), camptothecin (PNU 166148) (both failed in clinical trials underlining the importance of rational design), and most recently HPMA-copolymer platinates (AP5280 and then AP5346-ProLindac(TM)) entered Phase II clinical development. There are a growing array of second generation HPMA copolymer-based systems involving combination therapy, incorporating putative targeting ligands, having an ever more complex architecture, and both drug and protein conjugates are being proposed as novel treatments for diseases other than cancer. Despite their promise, and the success of polymeric drugs and polymer-protein conjugates, no polymer-drug conjugate (HPMA copolymer-based or otherwise) has yet entered routine clinical use. It is timely to reflect on the progress made over the last 30 years, the relative merits of HPMA copolymers as a platform compared to other polymeric carriers, and comment on their future potential as polymer-based nanomedicines into the 21st century in comparison with the many alternative strategies now emerging for creation of nanopharmaceuticals.
N-(2-Hydroxypropyl)methacrylamide (HPMA) copolymer conjugates containing doxorubicin designed in the late 1970s/early 1980s as anticancer polymer therapeutics were the first synthetic polymer-based anticancer conjugates to enter clinical trial beginning in 1994. Early clinical results were promising, confirming activity in chemotherapy refractory patients and the safety of HPMA copolymers as a new polymer platform in this setting. Subsequent Phase I/II trials have investigated conjugates containing paclitaxel (PNU 166945), camptothecin (PNU 166148) (both failed in clinical trials underlining the importance of rational design), and most recently HPMA-copolymer platinates (AP5280 and then AP5346-ProLindac(TM)) entered Phase II clinical development. There are a growing array of second generation HPMA copolymer-based systems involving combination therapy, incorporating putative targeting ligands, having an ever more complex architecture, and both drug and protein conjugates are being proposed as novel treatments for diseases other than cancer. Despite their promise, and the success of polymeric drugs and polymer-protein conjugates, no polymer-drug conjugate (HPMA copolymer-based or otherwise) has yet entered routine clinical use. It is timely to reflect on the progress made over the last 30 years, the relative merits of HPMA copolymers as a platform compared to other polymeric carriers, and comment on their future potential as polymer-based nanomedicines into the 21st century in comparison with the many alternative strategies now emerging for creation of nanopharmaceuticals.
Greco, F. and Vicent, M.J.*, Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev, 2009. 61(13): p. 1203-13. [PubMed]
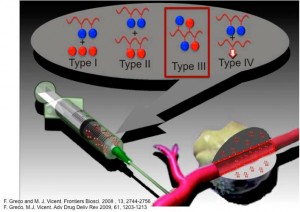 The discovery of new molecular targets and the subsequent development of novel anticancer agents are opening new possibilities for drug combination therapy as anticancer treatment. Polymer-drug conjugates are well established for the delivery of a single therapeutic agent, but only in very recent years their use has been extended to the delivery of multi-agent therapy. These early studies revealed the therapeutic potential of this application but raised new challenges (namely, drug loading and drugs ratio, characterisation, and development of suitable carriers) that need to be addressed for a successful optimisation of the system towards clinical applications.
The discovery of new molecular targets and the subsequent development of novel anticancer agents are opening new possibilities for drug combination therapy as anticancer treatment. Polymer-drug conjugates are well established for the delivery of a single therapeutic agent, but only in very recent years their use has been extended to the delivery of multi-agent therapy. These early studies revealed the therapeutic potential of this application but raised new challenges (namely, drug loading and drugs ratio, characterisation, and development of suitable carriers) that need to be addressed for a successful optimisation of the system towards clinical applications.
Vicent, M.J.*, Ringsdorf, H., and Duncan, R., Polymer therapeutics: clinical applications and challenges for development. Adv Drug Deliv Rev, 2009. 61(13): p. 1117-20. [PubMed]
 This preface is part of the Advanced Drug Delivery Reviews theme issue on “Polymer Therapeutics: Clinical Applications and Challenges for Development”.
This preface is part of the Advanced Drug Delivery Reviews theme issue on “Polymer Therapeutics: Clinical Applications and Challenges for Development”.
Vicent, M.J., Dieudonne, L., Carbajo, R.J., and Pineda-Lucena, A., Polymer conjugates as therapeutics: future trends, challenges and opportunities. Expert Opin Drug Deliv, 2008. 5(5): p. 593-614. [PubMed]
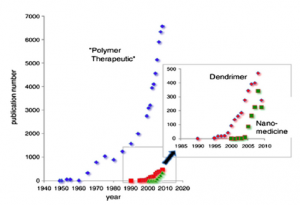
 Clinical proof of concept for polymer conjugates has already been achieved over the last 30 years, with a family of polymer-protein conjugates reaching the market and an exponentially growing list of polymer-drug conjugates currently in clinical trials. However, many challenges and opportunities still lie ahead, providing scope to develop this platform technology further. METHODS: The delivery of new anticancer agents aimed at novel molecular targets and their combination, the development of both new polymeric materials with defined architectures and the treatment of diseases other than cancer are the most exciting and promising areas. The latest advances and future trends in the polymer conjugate field will be presented in this article, providing an insight into their potential in the clinics and offering a wide range of research approaches within the scientific community. RESULTS/CONCLUSION: Polymer therapeutics is a rapidly emerging field with exponentially growing opportunities to achieve medical treatments with highly enhanced therapeutic value.
Clinical proof of concept for polymer conjugates has already been achieved over the last 30 years, with a family of polymer-protein conjugates reaching the market and an exponentially growing list of polymer-drug conjugates currently in clinical trials. However, many challenges and opportunities still lie ahead, providing scope to develop this platform technology further. METHODS: The delivery of new anticancer agents aimed at novel molecular targets and their combination, the development of both new polymeric materials with defined architectures and the treatment of diseases other than cancer are the most exciting and promising areas. The latest advances and future trends in the polymer conjugate field will be presented in this article, providing an insight into their potential in the clinics and offering a wide range of research approaches within the scientific community. RESULTS/CONCLUSION: Polymer therapeutics is a rapidly emerging field with exponentially growing opportunities to achieve medical treatments with highly enhanced therapeutic value.
Greco, F. and Vicent, M.J.*, Polymer-drug conjugates: current status and future trends. Front Biosci, 2008. 13: p. 2744-56. [PubMed]
 Polymer conjugates are nano-sized, multi-component constructs already in the clinic as anticancer compounds, both as single agents or as elements of combinations. They have the potential to improve pharmacological therapy of a variety of solid tumors. Polymer-drug conjugation promotes passive tumor targeting by the enhanced permeability and retention (EPR) effect and allows for lysosomotropic drug delivery following endocytic capture. In the first part of this review, we analyze the promising results arising from clinical trials of polymer-bound chemotherapy. The experience gained on these studies provides the basis for the development of a more sophisticated second-generation of polymer conjugates. However, many challenges still lay ahead providing scope to develop and refine this field. The ”technology platform” of polymer therapeutics allows the development of both new and exciting polymeric materials, the incorporation of novel bioactive agents and combinations thereof to address recent advances in drug therapy. The rational design of polymer drug conjugates is expected to realize the true potential of these “nanomedicines”.
Polymer conjugates are nano-sized, multi-component constructs already in the clinic as anticancer compounds, both as single agents or as elements of combinations. They have the potential to improve pharmacological therapy of a variety of solid tumors. Polymer-drug conjugation promotes passive tumor targeting by the enhanced permeability and retention (EPR) effect and allows for lysosomotropic drug delivery following endocytic capture. In the first part of this review, we analyze the promising results arising from clinical trials of polymer-bound chemotherapy. The experience gained on these studies provides the basis for the development of a more sophisticated second-generation of polymer conjugates. However, many challenges still lay ahead providing scope to develop and refine this field. The ”technology platform” of polymer therapeutics allows the development of both new and exciting polymeric materials, the incorporation of novel bioactive agents and combinations thereof to address recent advances in drug therapy. The rational design of polymer drug conjugates is expected to realize the true potential of these “nanomedicines”.
Vicent, M.J., Perez-Paya, E., and Orzaez, M., Discovery of inhibitors of protein-protein interactions from combinatorial libraries. Curr Top Med Chem, 2007. 7(1): p. 83-95. [PubMed]
 Protein-protein interactions play a central role within numerous processes in the cell. The relevance of the processes in which this type of interactions are implicated make them responsible for many pathological situations. In the last decade protein-protein interfaces have shown their potential as new drug targets, and combinatorial chemistry has been defined as a useful tool in this line. This review gives a global vision of the actual situation of combinatorial chemistry, highlighting its applicability to high-throughput drug discovery and giving some crucial examples of its contribution to find modulators of protein-protein interactions.
Protein-protein interactions play a central role within numerous processes in the cell. The relevance of the processes in which this type of interactions are implicated make them responsible for many pathological situations. In the last decade protein-protein interfaces have shown their potential as new drug targets, and combinatorial chemistry has been defined as a useful tool in this line. This review gives a global vision of the actual situation of combinatorial chemistry, highlighting its applicability to high-throughput drug discovery and giving some crucial examples of its contribution to find modulators of protein-protein interactions.
Vicent, M.J.*, Polymer-drug conjugates as modulators of cellular apoptosis. AAPS J, 2007. 9(2): p. E200-7. [PubMed]
The successful clinical application of polymer-protein conjugates (PEGylated enzymes and cytokines) and the promising results arising from clinical trials with polymer-bound chemotherapy (eg, doxorubicin or paclitaxel) have established their potential to reduce toxicity and improve activity in chemotherapy-refractory patients. Furthermore, and more important, they have also provided a firm foundation for more sophisticated second-generation constructs that deliver the newly emerging target-directed bioactive agents (eg, modulators of apoptosis, cell cycle, anti-angiogenic drugs) in addition to polymer-based drug combinations (eg, endocrine therapy and chemotherapy). This review will focus on polymer-drug conjugate modulators of cellular apoptosis to be used as single pro-apoptotic (eg, cancer) or anti-apoptotic (eg, ischemia) agents or as a combination therapy.
Orzáez, M., Mora, P., Mondragón, L., Pérez-Payá, E., and Vicent, M.J.*, Solid-phase Chemistry: A Useful Tool to Discover Modulators of Protein Interactions. International Journal of Peptide Research and Therapeutics, 2007. 13(1): p. 281-293. [Link]
The Solid phase synthesis (SPS) concept, first developed for biopolymers, has spread in every field where organic synthesis is involved. While the potential of the solid-phase method was obvious in 1959 to its discoverer, Prof. R. B. Merrifield, it was unpredictable its dominance in peptide synthesis and especially in combinatorial chemistry, an area not yet conceived. SPS paved the way for solid-phase combinatorial approaches as many laboratories and companies focused on the development of technologies and chemistry suitable to this new methodology. This resulted in the spectacular outburst of combinatorial chemistry, which profoundly changed the approach for new drug discovery. Combinatorial chemistry is currently considered a valid approach for a wide range of biomedical applications, such as, target validation and drug discovery.
Vicent, M.J.* and Duncan, R., Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol, 2006. 24(1): p. 39-47. [PubMed]
Interdisciplinary research at the interface of polymer chemistry and the biomedical sciences has produced the first polymer-based nanomedicines for the diagnosis and treatment of cancer. These water-soluble hybrid constructs, designed for intravenous administration, fall into two main categories: polymer-protein conjugates or polymer-drug conjugates. Polymer conjugation to proteins reduces immunogenicity, prolongs plasma half-life and enhances protein stability. Polymer-drug conjugation promotes tumor targeting through the enhanced permeability and retention (EPR) effect and, at the cellular level following endocytic capture, allows lysosomotropic drug delivery. The successful clinical application of polymer-protein conjugates (PEGylated enzymes and cytokines) and promising results arising from clinical trials with polymer-bound chemotherapy (e.g. doxorubicin, paclitaxel, camptothecins) has provided a firm foundation for more sophisticated second-generation constructs that deliver the newly emerging target-directed anticancer agents (e.g. modulators of the cell cycle, signal transduction inhibitors and antiangiogenic drugs) in addition to polymer-drug combinations (e.g. endocrine- and chemo-therapy).
Duncan, R., Vicent, M.J., Greco, F., and Nicholson, R.I., Polymer-drug conjugates: towards a novel approach for the treatment of endocrine-related cancer. Endocr Relat Cancer, 2005. 12 Suppl 1: p. S189-99. [PubMed]
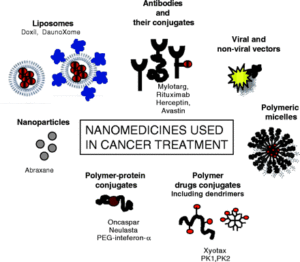 The last decade has seen successful clinical application of polymer-protein conjugates (e.g. Oncaspar, Neulasta) and promising results in clinical trials with polymer-anticancer drug conjugates. This, together with the realisation that nanomedicines may play an important future role in cancer diagnosis and treatment, has increased interest in this emerging field. More than 10 anticancer conjugates have now entered clinical development. Phase I/II clinical trials involving N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin (PK1; FCE28068) showed a four- to fivefold reduction in anthracycline-related toxicity, and, despite cumulative doses up to 1680 mg/m2 (doxorubicin equivalent), no cardiotoxicity was observed. Antitumour activity in chemotherapy-resistant/refractory patients (including breast cancer) was also seen at doxorubicin doses of 80-320 mg/m2, consistent with tumour targeting by the enhanced permeability (EPR) effect. Hints, preclinical and clinical, that polymer anthracycline conjugation can bypass multidrug resistance (MDR) reinforce our hope that polymer drugs will prove useful in improving treatment of endocrine-related cancers. These promising early clinical results open the possibility of using the water-soluble polymers as platforms for delivery of a cocktail of pendant drugs. In particular, we have recently described the first conjugates to combine endocrine therapy and chemotherapy. Their markedly enhanced in vitro activity encourages further development of such novel, polymer-based combination therapies. This review briefly describes the current status of polymer therapeutics as anticancer agents, and discusses the opportunities for design of second-generation, polymer-based combination therapy, including the cocktail of agents that will be needed to treat resistant metastatic cancer.
The last decade has seen successful clinical application of polymer-protein conjugates (e.g. Oncaspar, Neulasta) and promising results in clinical trials with polymer-anticancer drug conjugates. This, together with the realisation that nanomedicines may play an important future role in cancer diagnosis and treatment, has increased interest in this emerging field. More than 10 anticancer conjugates have now entered clinical development. Phase I/II clinical trials involving N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin (PK1; FCE28068) showed a four- to fivefold reduction in anthracycline-related toxicity, and, despite cumulative doses up to 1680 mg/m2 (doxorubicin equivalent), no cardiotoxicity was observed. Antitumour activity in chemotherapy-resistant/refractory patients (including breast cancer) was also seen at doxorubicin doses of 80-320 mg/m2, consistent with tumour targeting by the enhanced permeability (EPR) effect. Hints, preclinical and clinical, that polymer anthracycline conjugation can bypass multidrug resistance (MDR) reinforce our hope that polymer drugs will prove useful in improving treatment of endocrine-related cancers. These promising early clinical results open the possibility of using the water-soluble polymers as platforms for delivery of a cocktail of pendant drugs. In particular, we have recently described the first conjugates to combine endocrine therapy and chemotherapy. Their markedly enhanced in vitro activity encourages further development of such novel, polymer-based combination therapies. This review briefly describes the current status of polymer therapeutics as anticancer agents, and discusses the opportunities for design of second-generation, polymer-based combination therapy, including the cocktail of agents that will be needed to treat resistant metastatic cancer.
Altava, B., Burguete, M.I., Garcı́a-Verdugo, E., Luis, S.V., Vicent, M.J., and Mayoral, J.A., Supported chiral catalysts: the role of the polymeric network. Reactive and Functional Polymers, 2001. 48(1–3): p. 25-35. [Link]
In the preparation of chiral-supported catalysts, the immobilization process can produce changes in the behavior of the resulting resin-supported species. The polymeric matrix plays important roles that affect the activity, selectivity and stability of the final catalysts. Pseudodilution effects very often favor the activity of the supported species, and the presence of the hydrophobic matrix increases the stability of the water-sensitive active sites. The steric interaction between the polymeric backbone and the groups in the chiral auxiliary can modify, even dramatically, the stereochemical outcome of the reaction.
